Abstract
A thorough endoscopic visualization of the digestive mucosa is essential for reaching an accurate diagnosis and to treat the different lesions. Standard white light endoscopes permit a good mucosa examination but, nowadays, the introduction of powerful endoscopic instrumentations increased ability to analyze the finest details. By applying dyes and zoom-magnification endoscopy further architectural detail of the mucosa can be elucidated. New computed virtual chromoendoscopy have further enhanced optical capabilities for the evaluation of submucosal vascolar pattern. Recently, confocal endomicroscopy and endocytoscopy were proposed for the study of ultrastructural mucosa details. Because of the technological contents of powerful instrumentation, a good knowledge of implemented technologies is mandatory for the endoscopist, nowadays. Nevertheless, there is a big confusion about this topic. We will try to explain these technologies and to clarify this terminology.
Keywords: HDTV, Zoom endoscopy, Magnifying endoscopy, Fujinon intelligent color enhancement, Narrow band imaging, I-scan, Confocal laser endoscopy, Endocytoscopy
INTRODUCTION
The need of an ever-growing earliness in detection of digestive malignant lesions has led the endoscopy onto a new high-technology course through the development of the so-called powerful endoscopy. Keeping pace with the rapid developments in technology, the optical features of these new powerful endoscopes offer a resolution that allows the visibility of new surface details.
The new powerful digestive endoscopy enables to perform high-resolution endoscopy, high-magnification endoscopy (magnifying or zooming), computed virtual chromoendoscopy (CVC), confocal laser endoscopy (CLE), and endocitoscopy.
Some of these techniques increase diagnostic performances through resolution improvement, while other techniques through modifying the chromatic spectrum of the endoscopic picture. In some cases the augmented vision is due to the charge coupled device (CCD) features, in some other is due to the features of the central processor unit (CPU).
Because of the specific contents of powerful instrumentation, a good knowledge of implemented technologies is mandatory for the endoscopist, nowadays. Nevertheless, there is a big confusion about the technological terminology, despite the number of papers on the matter that are, sometimes, inaccurate. In this editorial, we will try to explain the technologies implemented in powerful endoscopes, clarifying some terms often abused, sometimes misused.
HIGH-RESOLUTION AND HIGH MAGNIFICATION ENDOSCOPY
The video capabilities of color images of standard definition (SD) endoscopes are based on traditional television (TV) broadcast formats[1,2]. The SD signals offer images in a 4:3 aspect ratio, with image resolutions of 640 to 700 pixels width by 480 to 525 pixels or ‘‘lines’’ height (approximately 367.000 pixels)[2]. SD endoscopes are equipped with CCD chips that produce an image signal of 100 000 to 400 000 pixels, which are displayed in the SD format. Advances in CCD technology have resulted in smaller CCDs with an increased number of pixels and increased resolution. The CCDs used in current so-called high-resolution or high-definition (HD) endoscopes produce signal images with resolutions ranging from 850 000 pixels to more than 1 million pixels.
A HD and high resolution image is generally defined as having a resolution higher than 650 to 720 lines (height)[3]. Moreover, images may be progressive or interlaced. With progressive (p) images, lines are scanned consecutively and the image is painted 60 times per second, whereas with interlaced (i) images, every other line is scanned and the image is painted in 2 passes at 30 times per second each.
HD video imaging can be displayed in either TV or computer monitor formats. The 16:9 aspect ratio is not useful to display images from round endoscopic lenses. Traditionally, endoscopic images are displayed in a 4:3 aspect ratio to match the standard aspect ratios of SD TV and because this ratio provides the highest pixel density and resolution possible given the lens shape. Display in computer monitor formats use progressive scanning and is not restricted by broadcast HD formats or aspect ratios. Monitors had traditionally 4:3 aspect ratios but recently 5:4 ratios have become more popular. Current high resolution endoscopic CCDs display images in either 4:3 or 5:4 aspect ratios[3].
It is important to recognize that, to provide a true HD image, each component of the system (e.g., the endoscope CCD, the processor, the monitor, and transmission cables) must be HD compatible. Three different high-resolution endoscope systems are currently commercially available: (1) Olympus high-resolution endoscopes are designed based on the commercial availability of TVs and recorders for output onto HDTVs. The output from the endoscope is enhanced to 1080i; however, the endoscopic image itself is displayed within a 1280 × 1024-pixel frame; (2) Fujinon high-resolution endoscopes are designed for output onto computer monitors. The first Fujinon CCD chips were 1077 × 788 pixels and their output was equivalent to XGA monitors[2]; however, current endoscopes have an output of 1280 × 960 pixels. The actual resolution of the CCD is proprietary. The latest processors enhance the image to 1080 i; and (3) Pentax Medical high-resolution endoscopes are designed for output onto computer monitors. The Pentax CCD provides 1280 × 1024 pixels and displays at native resolution.
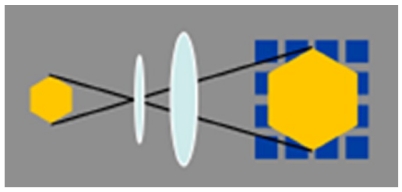
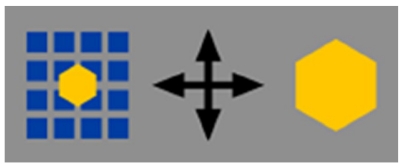
High-resolution endoscopes magnify the endoscopic images 30 to 35 times. Zoom endoscopes are defined by the capacity to perform optical zoom by using a movable lens in the tip of the endoscope[4]. The optical zoom provides a closer image of the target while maintaining image display resolution (Figure 1). This is distinguished from electronic magnification, which simply moves the image closer on the display and results in a decreased number of pixels composing the area of display, with no improvement in resolution (Figure 2)[5]. With a suitable processor, conventional endoscopes provide an electronic magnification of 1.5 to 2. Although standard endoscopes magnify images 30 to 35 times, zoom endoscopes can optically magnify images up to 150 times, depending on the size of the monitor.
Figure 1.
Zoom endoscopes perform optical zoom by using a movable lens in the tip of the endoscope: This system provides a closer image of the target while maintaining image display resolution.
Figure 2.
Electronic magnification simply moves the image closer on the display and results in a decreased number of pixels composing the area of display, with no improvement in resolution.
COMPUTED VIRTUAL CHROMOENDOSCOPY
CVC is a real-time, on-demand endoscopic imaging technique that, adjusting the spectroscopic characteristics of the videoendoscopic systems through a frame sequential lighting method[6], allows to enhance visualization of the vascular network and mucosal surface texture in an effort to improve tissue characterization, differentiation, and diagnosis. CVC is considered a potential alternative to traditional chromoendoscopy, providing contrast enhancement of tissue surface structures, although it has not been studied as extensively as chromoendoscopy. Three different CVC systems are now commercially available: the Olympus Narrow Band Imaging (NBI), the Fujinon Intelligent Color Enhancement (FICE), and the Pentax iScan.
Standard videoendoscope systems use the entire spectrum of visible light (400-700 nm). These white-light imaging endoscopic systems are designed to simulate daylight, thus allowing examining the tissues in their natural colors. This kind of videoendoscopic images can be obtained by one of two different systems: red-green-blue (RGB) sequential and color CCD[7].
In the RGB sequential system, the light from a xenon arc lamp is filtered through a rotating broadband RGB filter located between the lamp and the endoscope’s light guide in order to obtain sequential bursts of red, green, and blue light that give rise to the visual strobe effect. After tissue illumination, the reflected red, green, and blue tissue images are sequentially captured by a monochromatic CCD at the tip of the endoscope and transmitted to a video processor. The 3 images are fed into the electron guns that illuminate respectively the red, green, and blue phosphor dots on the monitor to create a final composite image in full natural color[8].
The color CCD system use a micromosaic color filter mounted over the CCD itself. Continuous white-light illumination from the xenon lamp is delivered to tissue by the endoscope’s light guide, and the reflected light and image created on the CCD surface is then processed by circuitry in the video processor before display. Similar to the RGB system, tissue structures that heavily reflect the red, green, and blue light are displayed on the R, G and B video channels on the video monitor, respectively[8].
The NBI system (Olympus Medical Systems, Tokyo, Japan) emphasizes the mucosal microvasculature and is able to identify vascular alterations indicating pathologic conditions[9-12]. In the NBI system, narrow bandpass filters are placed in front of a conventional white-light source in order to produce a contrast between vascular structures and the surrounding mucosa.
When compared to the initial 3-band NBI prototypes, currently available NBI systems use just 2 different narrow band filters[8]: (1) The first filter provides tissue illumination in the blue spectrum of light at 415 nm, emphasizing capillaries in the superficial mucosal layer and showing them in brown; and (2) The second filter provides tissue illumination in the green spectrum of light at 540 nm; this wave-length corresponds to the secondary hemoglobin absorption peak, and emphasizes deeper mucosal and submucosal venular vessels displaying them in cyan.
The NBI system can be coupled with electronic or optical (zoom) magnification for enhanced visualization of mucosal details. The FICE system (Fujinon, Saitama, Japan) is merchandised as a digital image processing technique enhancing the mucosal surface structures by using selected wavelengths of light in reconstituted virtual images.
Unlike NBI (that uses optical filters), the FICE system is software-driven and uses an image processing algorithm that is based on spectral estimation methods. In this technology, developed by professor Yoichi Miyake[13], a standard image captured by a color CCD videoendoscope is sent to a spectral estimation matrix processing circuit contained in the EPX 4400 video processor. Here, the various pixels spectra corresponding to the conventional image are mathematically estimated. Because the pixels spectra are well known, it is possible to implement imaging on a single wavelength. Such single-wavelength images are randomly selected, and assigned to red, green and blue respectively, to build and display a CVC-enhanced color image. The digital processing system is able to switch between an ordinary image to a FICE image immediately simply pressing a button on the handle of the endoscope. It is possible to select the most suitable wavelengths for examination because of the system’s variable setting functions, with up to ten preselect settings.
These ten presets can also be customized and configured from a very large number of wavelength permutations, because any of 60 wavelengths (400 to 695 nm, in increments of 5 nm) can be input into any of the 3 (RGB) channels[14]. A push button on the handle of the endoscope can be programmed to enable switching between the conventional white-light image and the corresponding FICE image of a single specified preset.
The FICE system can also be coupled with electronic or optical (zoom) magnification for enhanced visualization of mucosal details. iScan (Pentax, Tokyo, Japan) is the latest CVC technology developed and is merchandised as a digital contrast method among endoscopic imaging techniques[15].
This CVC system has three modes of image enhancement: (1) Surface Enhancement (SE) enhances the structures through recognition of the edges; (2) Contrast Enhancement (CE) enhances the depressed areas and differences in structure through colored presentation of low density areas; and (3) Tone Enhancement (TE) enhances individual organs through modification of the combination of RGB components for each pixel.
SE and CE are possible switching between three enhancement levels (low, medium and high). TE is possible switching between three objects (esophagus, stomach and colon). The three modes (SE, CE and TE) are arranged in series, therefore, it is possible to apply two or more of these three modes at one time. Switching the levels or modes of enhancements can be done on a real-time basis, without any time lag by pushing a button, thus enabling an efficient endoscopic observation[16].
With SE, the difference in luminance intensity between the pixels concerned and the surrounding pixels is analyzed and the edge components are enhanced. With ordinary enhancement, minor changes in structure can be perceived as noise. Adjustment of the noise erasure function allows more evident enhancement of the edges[17]. When compared to normal images, SE images do not differ in brightness and differ little in color.
With CE, areas of lower luminance intensity are compared to surrounding pixels and identified on the basis of pixel-wise luminance intensity data, followed by relative enhancement of the B component through the slight suppression of R and G components in the low luminance area. As a result of CE, the low luminance area is stained in slightly bluish white, and minute irregularities on the mucosal surface are enhanced[18]. This images processing do not cause a change in image brightness or a marked change in the color of the images. It causes only a slight bluish-white staining of depressed areas.
With TE, the RGB components of the endoscope image are disintegrated into each component (R, G and B) which are converted independently along the tone curve, followed by a re-synthesis of the three components in order to yield a reconstructed image. The tone curve is depicted by plotting input (on the x axis) against output (on the y axis). The tone curve can be changed into various forms, by modifying of the parameters, into S and J types. If the tone curve assumes an S type, the high R-component area is shifted to a further higher range of R to enhance the color tone R, or the low R-component area is shifted to a further lower range of R to elevate the sensitivity to GB components, thus allowing clear enhancement of the differences in color tone. If the tone curve assumes a J-type form, the R component is shifted completely to a low R range, to elevate the overall sensitivity to GB components and the brightness/darkness contrast[16-19].
In conclusion, there have been no reported complications attributed to the use of NBI, FICE or iScan[8]. The costs of endoscope systems supplied with CVC is higher than those with white light but no formal cost analyses have been reported on this topic. Moreover, there are no unique CPT* (Current Procedural Terminology) codes for NBI, FICE or iScan[8].
CONFOCAL LASER ENDOSCOPY
CLE is a new endoscopic technology, developed to obtain high-resolution images of gastrointestinal mucosa, based on tissue illumination with a low-power laser with subsequent detection of the fluorescence light reflected from the tissue through a pinhole[20]. The term confocal refers to the alignment of both illumination and collection systems in the same focal plane[21]. The laser light is focused at a selected depth in the tissue of interest and reflected light is then refocused onto the detection system by the same lens. Only the returning light refocused through the pinhole is detected. The light reflected and scattered at other geometric angles from the illuminated object or refocused out of plane with the pinhole is excluded from detection[22]. This dramatically increases the image resolution, providing almost a histological examination, a kind of optical biopsy, of the superficial layer of the digestive tract[23-25]. Confocal imaging can be based on tissue reflectance or tissue fluorescence. The confocal devices based on tissue reflectance do not require any contrast agents, but have many technical problems and low resolution, compromising clinical utility[24,26]. Whereas, confocal endomicroscopy based on tissue fluorescence uses local and/or intravenous contrast agents and generates high-quality images comparable with traditional histology[27,28].
Two kind of confocal endoscopes are today commercially available. The first model is integrated into the distal tip of a conventional upper endoscope (EG-3870CIK; Pentax, Tokyo, Japan) or colonoscope (EC-3870CILK; Pentax). The second type uses a dedicated confocal miniprobe with laser microscope (Mauna Kea Technologies, Paris, France) inserted through the accessory channel of a traditional endoscope. All these instruments have CE code and US Food and Drug Administration authorization and provide different depths of imaging, field of views, and lateral resolutions.
The Mauna Kea confocal gastro-intestinal miniprobes include CholangioFlex, GastroFlex (standard and UHD) and ColoFlex (standard and UHD). All probes generate dynamic images, with 12 frames per second and are reusable approximately for 20 studies. The depth of imaging is 40 mm to 70 mm for CholangioFlex probes, 70 to 130 mm for GastroFlex and ColoFlex probes, and 55 to 65 mm for GastroFlexUHD and ColoFlexUHD probes. The maximal field of view is 325 mm for CholangioFlex probes, 600 mm for GastroFlex and ColoFlex probes, and 240 mm for GastroFlexUHD and ColoFlexUHD probes. The lateral resolution is 3.5 mm for CholangioFlex, GastroFlex, and ColoFlex probes, and 1 mm for GastroFlexUHD and ColoFlexUHD[29,30].
The Pentax confocal microscope integrated into the conventional endoscopes acquires images at a scan rate of 1.6 frames per second (1024 × 512 pixels) or 0.8 frames per second (1024 × 1024 pixels) with an adjustable depth of scanning ranging from 0 mm to 250 mm, a field of view of 475 × 475 mm, a lateral resolution of 0.7 mm, and an axial resolution of 7 mm[31,32].
The fluorescent contrasts for CLE can be administered intravenously or topically. Intravenous fluorescein (Pharmalab, Lane Cove, New South Wales, Australia) distributes throughout the extracellular matrix of the surface epithelium and lamina propria but does not stain cell nuclei[21]. Topically administered acriflavin (Sigma Pharmaceuticals, Clayton, Victoria, Australia), tetracicline or cresyl violet (AnaSpec, Inc, San Jose, CA, United States) stains cell nuclei of the surface epithelium but does not penetrate to deeper layers of the mucosa[21]. Acriflavin is a mutagenic dye and a potential human carcinogen, which will likely limit its clinical utility[33]. After the contrast administration, the tip of the confocal endomicroscope or miniprobe is positioned in gentle contact with the area of interest to obtain high-resolution confocal images. Accumulated images can be saved for postprocedural analysis.
ENDOCYTOSCOPY
Endocytoscopy (EC) is an ultra magnification technique providing images of surface epithelial structures at cellular resolution[34-36]. This technique is based on the contact light microscopy principle leading to real-time visualization of the cellular structures of the superficial epithelial layer. The technology uses a fixed-focus, high-power objective lens projecting onto a CCD very highly magnified images from a 0.5 mm diameter sample.
Currently we have two kind of EC instruments, both manufactured by Olympus (Tokyo, Japan) and available only as prototype devices: (1) The probe-based instrument consists of 2 flexible devices providing ultra-high magnification images of the epithelial surface at 570x or 1400x on a 19-inch monitor (or 450x and 1125x on a 14-inch monitor); these probes are realized to fit through therapeutic channel of endoscopes (minimum 3.7 mm) and necessitate contact with the tissue surface for imaging[37]; and (2) The endoscope-based instrument integrates the EC component within the endoscope. Both the upper (103 cm long) and lower (133 cm long) prototype provide an image magnification of 580x on a 19-inch monitor, in addition to having conventional optical magnification and narrow-band imaging capabilities. The tip of the endoscopes is placed in contact with the tissue surface to generate endocytoscopic images[38].
Endocytoscopic visualization necessitates treatment of the mucosa with a mucolytic agent, such as N-acetylcysteine, and prestaining with an absorptive agent, such as methylene blue (0.5%-1%) or toluidine blue (0.25%). Excess staining is washed off before imaging[39]. EC-based image criteria for tissue diagnosis and/or classification in the esophagus, stomach, and colon have been described, but not yet validated prospectively[40-42].
CONCLUSION
The frontiers of endoscopy continue to widen: the development and the implementation of new technologies in endoscopic instrumentation is a challenge that we seem to have won. Nevertheless, as the experience teaches us, not all of these technologies will be ultimately integrated into the practice of digestive endoscopy. Some technologies are still experimental or in the proof-of-concept stage, and some will have a story of failure or prolonged stagnation of promising concepts. Just a few will become viable, showing an important impact on diagnosis and treatment of digestive diseases.
However there is no doubt that this new course of gastro-intestinal endoscopy requires knowledge and mastery of implemented technologies and their specific terms. The new endoscopist will probably find himself closer to the engineering, IT, and technical environment.
We are sure that our professional category, the scientific societies and journals may play an important role in organizing a formalized program aimed at supporting the approach to technological innovation in endoscopy.
Footnotes
Peer reviewer: Nevin Oruc, MD, Associate Professor, Gastroenterology Department, Ege University, Ege University Hospital, Bornova, İzmir 35100, Turkey
S- Editor Yang XC L- Editor A E- Editor Yang XC
References
- 1.Mårvik R, Langø T. High-definition television in medicine. Surg Endosc. 2006;20:349–350. doi: 10.1007/s00464-005-0826-x. [DOI] [PubMed] [Google Scholar]
- 2.Udagawa T, Amano M, Okada F. Development of magnifying video endoscopes with high resolution. Dig Endosc. 2001;13:163–169. doi: 10.1046/j.1443-1661.2001.00128.x. [DOI] [Google Scholar]
- 3.Tanaka S, Kaltenbach T, Chayama K, Soetikno R. High-magnification colonoscopy (with videos) Gastrointest Endosc. 2006;64:604–613. doi: 10.1016/j.gie.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Bruno MJ. Magnification endoscopy, high resolution endoscopy, and chromoscopy; towards a better optical diagnosis. Gut. 2003;52 Suppl 4:iv7–i11. doi: 10.1136/gut.52.suppl_4.iv7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon RS, Adler DG, Chand B, Conway JD, Diehl DL, Kantsevoy SV, Mamula P, Rodriguez SA, Shah RJ, Wong Kee Song LM, et al. High-resolution and high-magnification endoscopes. Gastrointest Endosc. 2009;69:399–407. doi: 10.1016/j.gie.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 6.Pohl J, May A, Rabenstein T, Pech O, Nguyen-Tat M, Fissler-Eckhoff A, Ell C. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007;39:594–598. doi: 10.1055/s-2007-966649. [DOI] [PubMed] [Google Scholar]
- 7.Bosco JJ, Barkun AN, Isenberg GA, Nguyen CC, Petersen BT, Silverman WB, Slivka A, Taitelbaum G, Ginsberg GG. Gastrointestinal endoscopes: May 2003. Gastrointest Endosc. 2003;58:822–830. doi: 10.1016/s0016-5107(03)02013-3. [DOI] [PubMed] [Google Scholar]
- 8.Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kwon R, Mamula P, Rodriguez B, Shah RJ, et al. Narrow band imaging and multiband imaging. Gastrointest Endosc. 2008;67:581–589. doi: 10.1016/j.gie.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–295. doi: 10.1016/s0016-5107(03)02532-x. [DOI] [PubMed] [Google Scholar]
- 11.Muto M, Katada C, Sano Y, Yoshida S. Narrow band imaging: a new diagnostic approach to visualize angiogenesis in superficial neoplasia. Clin Gastroenterol Hepatol. 2005;3:S16–S20. doi: 10.1016/s1542-3565(05)00262-4. [DOI] [PubMed] [Google Scholar]
- 12.Kuznetsov K, Lambert R, Rey JF. Narrow-band imaging: potential and limitations. Endoscopy. 2006;38:76–81. doi: 10.1055/s-2005-921114. [DOI] [PubMed] [Google Scholar]
- 13.Miyake Y, Nakaguchi T, Tsumura N, Yamataka S. Development of new electronic endoscopes using the spectral images of an internal organ. Ariz, 2005. November 7-11; Scottsdale. In: Proceedings of the IS&T/SID’s Thirteen Color Imaging Conference;; 2005. pp. 261–263. [Google Scholar]
- 14.Burgos H, Porras M, Brenes F, Izquierdo E. Fujinon FICE Electronic Chromovideoendoscopy Helps Differentiate the Type of Metaplasia in Patients with Chronic Atrophic Gastritis. Gastrointest Endosc. 2007;65:AB 353. [Google Scholar]
- 15.Tajiri H, Niwa H. Proposal for a consensus terminology in endoscopy: how should different endoscopic imaging techniques be grouped and defined? Endoscopy. 2008;40:775–778. doi: 10.1055/s-2008-1077507. [DOI] [PubMed] [Google Scholar]
- 16.Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol. 2010;16:1043–1049. doi: 10.3748/wjg.v16.i9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetz M, Kiesslich R. Advanced imaging of the gastrointestinal tract: research vs. clinical tools? Curr Opin Gastroenterol. 2009;25:412–421. doi: 10.1097/MOG.0b013e32832d62c1. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman A, Kagel C, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis. 2010;42:45–50. doi: 10.1016/j.dld.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman A, Basting N, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High-definition endoscopy with i-Scan and Lugol’s solution for more precise detection of mucosal breaks in patients with reflux symptoms. Endoscopy. 2009;41:107–112. doi: 10.1055/s-0028-1119469. [DOI] [PubMed] [Google Scholar]
- 20.Wang TD. Confocal microscopy from the bench to the bedside. Gastrointest Endosc. 2005;62:696–697. doi: 10.1016/j.gie.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva BM. Pregnancy during residency: a look at the issues. J Am Med Womens Assoc. 1992;47:71–74. [PubMed] [Google Scholar]
- 22.Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kaul V, Kethu SR, Kwon RS, Mamula P, Rodriguez SA, et al. Confocal laser endomicroscopy. Gastrointest Endosc. 2009;70:197–200. doi: 10.1016/j.gie.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang TD, Van Dam J. Optical biopsy: a new frontier in endoscopic detection and diagnosis. Clin Gastroenterol Hepatol. 2004;2:744–753. doi: 10.1053/S1542-3565(04)00345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida S, Tanaka S, Hirata M, Mouri R, Kaneko I, Oka S, Yoshihara M, Chayama K. Optical biopsy of GI lesions by reflectance-type laser-scanning confocal microscopy. Gastrointest Endosc. 2007;66:144–149. doi: 10.1016/j.gie.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 25.Aisenberg J. Gastrointestinal endoscopy nears “the molecular era”. Gastrointest Endosc. 2008;68:528–530. doi: 10.1016/j.gie.2008.03.1075. [DOI] [PubMed] [Google Scholar]
- 26.Inoue H, Cho JY, Satodate H, Sakashita M, Hidaka E, Fukami S, Kazawa T, Yoshida T, Shiokawa A, Kudo S. Development of virtual histology and virtual biopsy using laser-scanning confocal microscopy. Scand J Gastroenterol Suppl. 2003:37–39. doi: 10.1080/00855910310001485. [DOI] [PubMed] [Google Scholar]
- 27.Sakashita M, Inoue H, Kashida H, Tanaka J, Cho JY, Satodate H, Hidaka E, Yoshida T, Fukami N, Tamegai Y, et al. Virtual histology of colorectal lesions using laser-scanning confocal microscopy. Endoscopy. 2003;35:1033–1038. doi: 10.1055/s-2003-44595. [DOI] [PubMed] [Google Scholar]
- 28.Kiesslich R, Neurath MF. Chromoendoscopy and other novel imaging techniques. Gastroenterol Clin North Am. 2006;35:605–619. doi: 10.1016/j.gtc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Becker V, Vercauteren T, von Weyhern CH, Prinz C, Schmid RM, Meining A. High-resolution miniprobe-based confocal microscopy in combination with video mosaicing (with video) Gastrointest Endosc. 2007;66:1001–1007. doi: 10.1016/j.gie.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 30.von Delius S, Feussner H, Wilhelm D, Karagianni A, Henke J, Schmid RM, Meining A. Transgastric in vivo histology in the peritoneal cavity using miniprobe-based confocal fluorescence microscopy in an acute porcine model. Endoscopy. 2007;39:407–411. doi: 10.1055/s-2007-966439. [DOI] [PubMed] [Google Scholar]
- 31.Sutin EL, Jacobowitz DM. Localization of substance P mRNA in cholinergic cells of the rat laterodorsal tegmental nucleus: in situ hybridization histochemistry and immunocytochemistry. Cell Mol Neurobiol. 1990;10:19–31. doi: 10.1007/BF00733632. [DOI] [PubMed] [Google Scholar]
- 32.Confocal chromoscopic endomicroscopy is superior to chromoscopy alone for the detection and characterisation of intraepithelial neoplasia in chronic ulcerative colitis (Gut 2008; 57: 196-204) Gut. 2008;57:1634. [PubMed] [Google Scholar]
- 33.Burleson GR, Caulfield MJ, Pollard M. Ozonation of mutagenic and carcinogenic polyaromatic amines and polyaromatic hydrocarbons in water. Cancer Res. 1979;39:2149–2154. [PubMed] [Google Scholar]
- 34.Kwon RS, Wong Kee Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Mamula P, et al. Endocytoscopy. Gastrointest Endosc. 2009;70:610–613. doi: 10.1016/j.gie.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Takubo K, Aida J, Sawabe M, Kurosumi M, Arima M, Fujishiro M, Arai T. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology. 2007;51:733–742. doi: 10.1111/j.1365-2559.2007.02766.x. [DOI] [PubMed] [Google Scholar]
- 36.Habbab MA, el-Sherif N. Recordings from the slow zone of reentry during burst pacing versus programmed premature stimulation for initiation of reentrant ventricular tachycardia in patients with coronary artery disease. Am J Cardiol. 1992;70:211–217. doi: 10.1016/0002-9149(92)91277-b. [DOI] [PubMed] [Google Scholar]
- 37.Kodashima S, Fujishiro M, Takubo K, Kammori M, Nomura S, Kakushima N, Muraki Y, Tateishi A, Kaminishi M, Omata M. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy. 2006;38:1115–1121. doi: 10.1055/s-2006-944915. [DOI] [PubMed] [Google Scholar]
- 38.Kumagai Y, Monma K, Kawada K. Magnifying chromoendoscopy of the esophagus: in-vivo pathological diagnosis using an endocytoscopy system. Endoscopy. 2004;36:590–594. doi: 10.1055/s-2004-814533. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee R, Reddy DN, Rao GV, Shekharan A, Ramji C. Application of high-resolution narrow band imaging and endocytoscopy for early diagnosis of esophageal neoplasia. Indian J Gastroenterol. 2008;27:204–206. [PubMed] [Google Scholar]
- 40.Sasajima K, Kudo SE, Inoue H, Takeuchi T, Kashida H, Hidaka E, Kawachi H, Sakashita M, Tanaka J, Shiokawa A. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006;63:1010–1017. doi: 10.1016/j.gie.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 41.Inoue H, Sasajima K, Kaga M, Sugaya S, Sato Y, Wada Y, Inui M, Satodate H, Kudo SE, Kimura S, et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006;38:891–895. doi: 10.1055/s-2006-944667. [DOI] [PubMed] [Google Scholar]
- 42.Eberl T, Jechart G, Probst A, Golczyk M, Bittinger M, Scheubel R, Arnholdt H, Knuechel R, Messmann H. Can an endocytoscope system (ECS) predict histology in neoplastic lesions? Endoscopy. 2007;39:497–501. doi: 10.1055/s-2007-966446. [DOI] [PubMed] [Google Scholar]