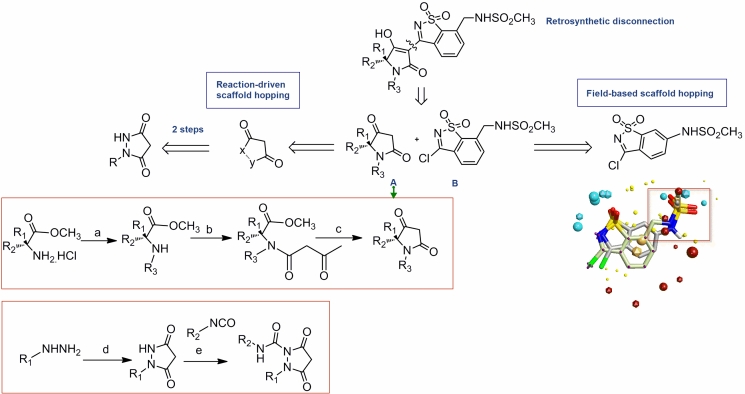
Figure 5.
Reaction-driven scaffold hopping. The disconnection approach resulted in two scaffolds A and B. A was modified using a simple scifinder substructure query limiting results on 2 steps synthetic pathways while B was modified using a fieldbased approach which was influenced too by the ease of synthesis and overall yield. Regarding synthesis of A: (a) for aromatic aldehydes :i—RCHO, TEA, MgSO4, THF, 25°C, 12 h; ii—NaBH4, MeOH, 25 °C, 1 h; for aliphatic aldehydes: RCHO, NaBH3CN, MeOH, 25°C, 12 h; (b) HO2CCH2CO2Et, EDC_HCl, TEA, DCM, 25 _C,12 h; (c) (i) NaOEt, EtOH, 25°C, 12 h; (ii) 1 M H2SO4 (aq), reflux, 1 h. Regarding synthesis of the new A analogue: (d) malonic acid,SOCl2 ;(e)RNCO,140°C, 10 min.