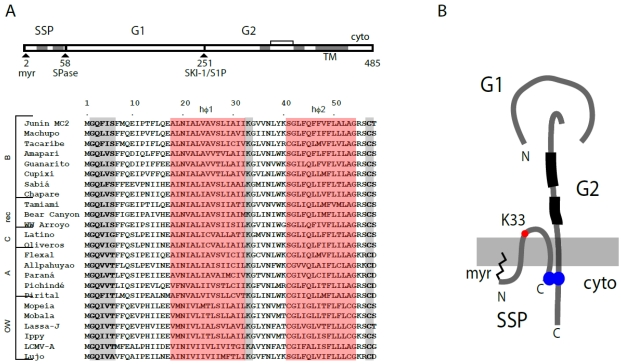
Figure 2.
Schematic representation of GPC open-reading frame, stable signal peptide (SSP) sequence alignment and the tripartite GPC protein complex. (A) The GPC open‑reading frame is diagrammed. Cleavage sites for signal peptidase (SPase) and subtilisin-like kexin protease-1/site-1-protease (SKI-1/S1P) are indicated, as are the mature SSP, G1 and G2 subunits. In SSP, the myristoylation at glycine 2 is marked, and the shaded regions denote the two hydrophobic regions (hɸ1 and hɸ2, highlighted in red in the sequence comparisons below). The transmembrane and cytoplasmic domains of G2 are indicated, as well as the two heptad‑repeat regions (shaded) and disulfide-bonded hinge region. The sequence comparison of SSP among arenaviruses is adapted from [32], in which accession numbers are listed. In addition to hɸ1 and hɸ2 (red), the conserved myristoylation motif, K33 and C57 residues are highlighted in gray. (B) Schematic drawing illustrating the subunit organization of the tripartite GPC complex. The membrane is shown in gray. SSP spans the membrane twice, and salient features are indicated: Membrane association of the myristoylated N-terminus, K33 in the SSP ectodomain, and the intersubunit zinc-binding motif that bridges the C‑terminal cytoplasmic domains of SSP and G2. The two heptad-repeat regions in the G2 ectodomain are depicted in black. The drawing is not to scale and the structural relationships among subunits is not known.