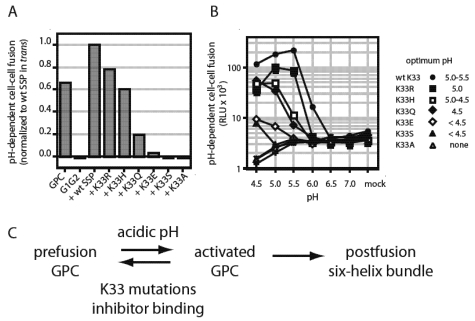
Figure 4.
K33 in SSP modulates pH-induced activation of membrane fusion. (A) Mutations that reduce positive polarity at SSP K33 were introduced into SSP and the membrane-fusion activity of the GPC complex was determined in our standard assay for cell-cell fusion. All SSPs were expressed in trans with the G1G2 precursor to reconstitute GPC, and fusion activity was normalized to that of the wild-type (K33) SSP. GPC and G1G2 indicate, respectively, the complete GPC open-reading frame and the precursor alone, in the absence of SSP. (B) The pH-dependence of the mutant GPC complexes was determined by varying the pH of the triggering pulse of acidic medium. The nominal optimal pH for each mutant is listed at right. (C) Model for pH-induced activation of GPC, and its inhibition by mutations at K33 and by small-molecule compounds. Acidic pH destabilizes the metastable prefusion GPC complex to favor an activated state, that spontaneously undergoes the class I structural reorganization resulting in formation of the six-helix bundle and membrane fusion. By contrast, K33 mutations and inhibitor binding disfavor the transition to the activated state. The molecular basis for maintaining the equilibrium between prefusion and activated states is unknown.