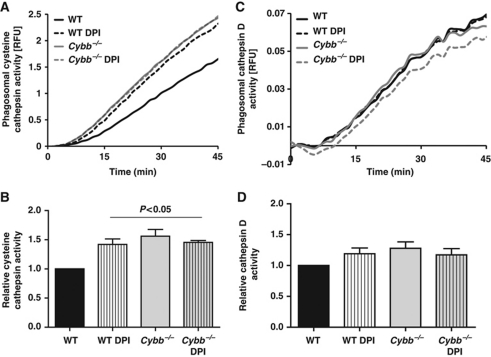
Figure 5.
NOX2 activity negatively affects activity of cysteine but not aspartic cathepsins in BMDC phagosomes. Relative phagosomal activities of cysteine (A, B) and aspartic (C, D) cathepsins were measured using cathepsin B/L and D/E peptide-based fluorogenic substrates [(biotin-LC-Phe-Arg)2-rhodamine 110: λex485 nm; λem520 nm and Mca-GKPILFFRLK(Dnp)-r-NH2: λex320 nm; λem405 nm] bound to IgG-opsonized particles, following phagocytosis by WT and Cybb−/− BMDCs, in the presence or absence of 0.5 μM DPI. (A, C) Representative traces of cathepsin B/L (A) and D/E (C) substrate hydrolysis relative to a calibration fluor Alexa Fluor 594 (λex544 nm; λem620 nm). (B, D) Average rates of substrate hydrolysis over five independent experiments relative to untreated controls. Rates were determined by calculation of the slope of the linear portion of the real-time traces (as described by y=mx+c, where y is the relative fluorescence, m is the slope and x is time) and expressed relative to DMSO-treated WT samples. Error bars represent s.e.m. P-values were calculated by ANOVA.