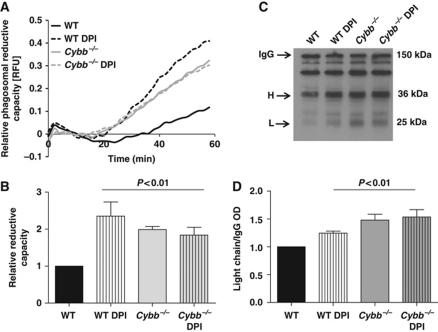
Figure 6.
NOX2 activity compromises the reductive capacity of phagosomes in BMDCs. Phagosomal reductive capacity was assessed by monitoring the ability of BMDC phagosomes to reduce mixed disulphides. (A, B) Reduction of a modified cystine-based fluorogenic substrate conjugated to an IgG-opsonized experimental particle following phagocytosis by WT and Cybb−/− BMDCs, in the presence or absence of 0.5 μM DPI. (A) Representative real-time traces of Bodipy FL L-cystine substrate reduction (λex485 nm; λem520 nm) relative to a calibration fluor Alexa Fluor 594 (λex544 nm; λem620 nm). (B) Average rates of substrate reduction over four independent experiments relative to untreated controls. Rates were determined by calculation of the slope of the linear portion of the real-time traces (as described by y=mx+c, where y is the relative fluorescence, m is the slope and x is time) and expressed relative to untreated WT samples. (C, D) Reduction of intermolecular disulphide bridges of IgG in BMDC phagosomes was determined by the degree of dissociation of light (L) and heavy (H) chains at 1 h following phagocytosis. BMDC phagosomes were allowed to phagocytose biotinylated IgG molecules used to opsonize BSA-conjugated experimental particles in the presence of protease inhibitors. Subunits were resolved by SDS–PAGE under non-reducing conditions and detected by western blotting. (C) Representative western blot image indicating relative dissociation of heavy (H) and light (L) chains from holo-IgG (IgG). (D) Average dissociation of the light chain relative to untreated controls over three independent experiments. Relative light chain dissociation was calculated by the density of the light chain band over the density of the holo-IgG band and expressed relative to DMSO-treated WT samples (B, D) Error bars denote s.e.m. P-values were calculated using ANOVA.