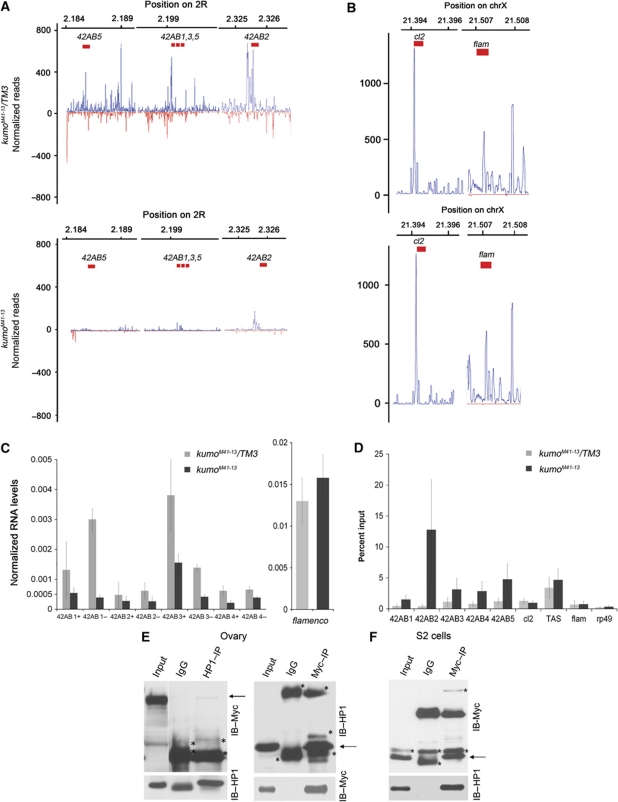
Figure 7.
The kumo mutation results in reduced cluster transcription and high HP1 occupancy at piRNA clusters. (A, B) Mapping of piRNAs to the indicated regions of the bidirectional cluster at 42AB and the unidirectional clusters at X (cluster 2 and flamenco), which were examined for the expression of the cluster transcripts and HP1 binding. In the kumo mutant ovaries, piRNA mapping to the bidirectional cluster were nearly eliminated, whereas no significant impact was observed in the number of piRNAs matching cluster 2 and flamenco. (C) Strand-specific quantitative RT–PCR showing the expression levels of cluster transcripts from the plus and minus strands (indicated by + and −, respectively) from the 42AB piRNA cluster. RNA levels from both strands in the kumo mutant ovaries are reduced compared with those in the heterozygous control. However, no significant difference in the expression levels of transcripts from a region of the flamenco piRNA cluster was observed between the control and kumo mutant ovaries. Error bars indicate standard error for three independent experiments. (D) Quantification of chromatin immunoprecipitation with anti-HP1 using primers at various regions of the 42AB piRNA cluster in control and kumo mutant ovaries. The percent input of immunoprecipitates is shown for each primer set. HP1 binding was enriched in kumo mutant ovaries compared with that in the kumo heterozygous controls with 1.5-, 12.8-, 3.1-, 2.8- and 4.8-fold increases at 42AB1–5, respectively. No significant increase was observed at unidirectional piRNA clusters, cluster 2 and flamenco, the controls, euchromatin region, rp49 and heterochromatin region (X-TAS) in the kumo mutant. Error bars indicate standard error from two independent experiments. (E) Co-immunoprecipitation of Myc–Kumo and HP1 from the ovarian lysates. Western blots for Myc–Kumo and HP1: 5% of input was loaded on the blots to detect HP1 in Myc–Kumo immunoprecipitate and in the reciprocal immunoprecipitation 1% of input was used. (F) HP1 is co-immunoprecipitated with Myc–Kumo in S2 cells. 1% input was used in the western blot. Asterisks denote the non-specific bands.