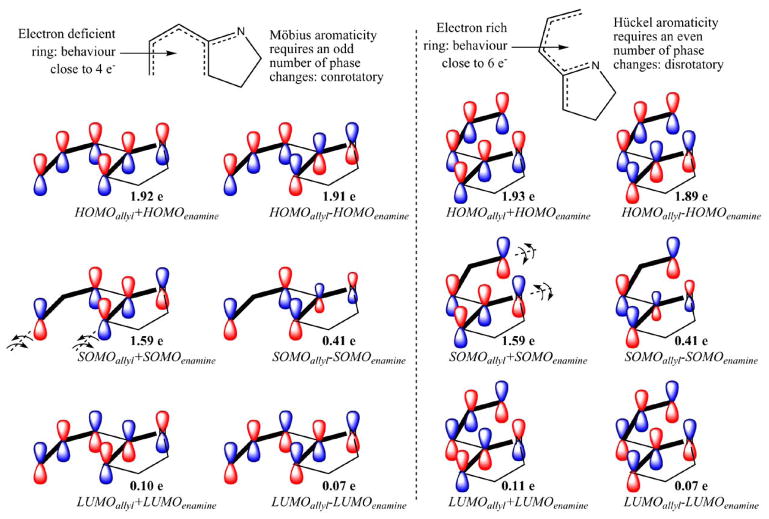
Fig. 6.
CASSCF natural orbitals of the active space of transition states for the C—C bond forming cyclization NC-6a (left) and C—N bond forming cyclization NC-6b (right). Bold lines indicate the disconnected ally and enamine radicals and the proposed allyl and enamine orbital combinations leading to each natural molecular orbital. Natural orbital populations are also provided to help quantify the diradical character inherent to these species (41% for both NC-6a and NC-6b, DR = [2 - n(HOMO)] · 100). The active space in these calculations includes the entire π system of the diyl intermediate resulting in a CASSCF(6,6)/6-31G(d) level of theory.