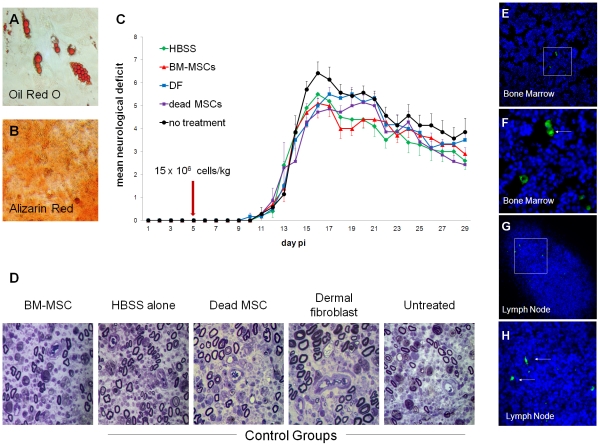
Figure 2. Lewis rat-derived MSCs do not improve clinical, electrophysiological or histological outcomes in experimental autoimmune neuritis.
(A, B) Apidogenic and osteogenic potential of CD90+/CD45−/CD11b− Lewis BM-MSCs. Adipogenesis confirmed by Oil red O staining and osteogenesis confirmed by Alizarin Red staining. (C) Clinical course of EAN following various treatments. HBSS = Hank's buffered saline solution vehicle alone, BM-MSCs = 15×106 bone marrow mesenchymal stem cells/kg, DF = 15×106 dermal fibroblasts/kg, dead MSCs = 15×106 dead bone marrow mesenchymal stem cells/kg, no treatment = no injection or anaesthesia. No significant differences in disease onset, severity or residual deficit were observed between groups. (D) Representative semithin sections from experimental groups. Inflammatory demyelination and axonal loss were observed in all groups. No histo-pathological differences were found between the treatment groups, as analysed in toluidine blue-stained micrographs of transverse sections (1 µm) through the sciatic nerves from animals in each group. (E–H) In cell tracing experiments, CFSE-labelled BM-MSCs were identified in bone marrow and lymphoid organs seven days after cell delivery (Hoechst – Blue; CFSE - Green; F & H show detail from E & G).