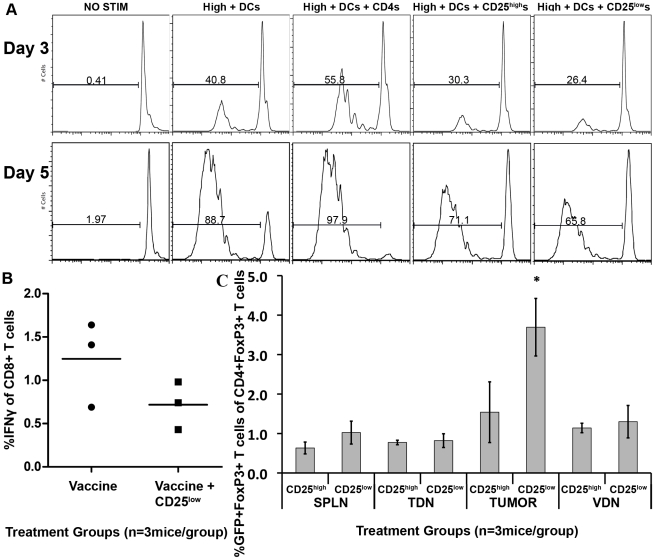
Figure 10. CD25lowFoxp3+ Tregs suppress high avidity T cells and traffic preferentially to the tumor.
(A) CD25low and CD25high CD4+ Treg cells suppress high avidity T cell activation in vitro. CD25low or CD25high CD4+ FoxP3gfp+, DC, and CFSE-labeled high avidity Thy1.2+ CD8+ neu-specific T cells were mixed as described in the Methods. CFSE dilution was measured on days 3 and 5. CD4+ T cells from FVB/N mice were used as controls. High+DCs = High avidity T cells+DCs. High+DCs+CD4s = High avidity T cells+DCs+FVB/N CD4+ T cells, (B) CD25lowCD4+FoxP3+ Tregs trend toward suppression in vivo. neu-N/FoxP3gfp mice were given tumor and vaccinated as in the Methods. CD4+CD25low Tregs were isolated 1 week later and transferred to FVB/N-TgN(TIE2GFP)287Sato mice which were then vaccinated. CD8+ T cells were isolated 2 weeks later and tested for the ability to secrete IFNγ in response to RNEU420–429. Data are presented as % RNEU420–429-specific, IFNγ+ of CD8+ T cells. This experiment was done twice with similar results. (C) CD25low Tregs traffic preferentially to the tumor. neu-N mice received tumor and one week later received Cy, vaccine, 6×106 high avidity T cells, and 5×105 CD25low or CD25high CD4+GFP+ Tregs. On day 5 CD25low or CD25high CD4+GFP+ Tregs recovered from tumors, spleens, TDNs, and VDNs were enumerated as a fraction of the total # of FoxP3+ T cells at each site (n = 3mice/group). * = p<0.05. This experiment was repeated three times with similar results.