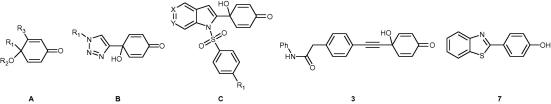
Table 1.
| Core | Compd | Structure |
EC50T. brucei (μM)a (SD, n) | EC50 MRC5 (μM)a (SD, n) | Spec. selectb | log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | X | Y | ||||||
| A | 1 | Ethynyl | H | H | n/a | n/a | 2.0 (0.80, 4) | 2.1 (0.15, 4) | 1 | 0.76 |
| A | 2 | Phenyl | H | H | n/a | n/a | 0.75d | 1.7d | 2 | 1.3 |
| n/a | 3 | n/a | n/a | n/a | n/a | n/a | 2.2 (0.0017, 2) | 0.67 (0.088, 2) | 0.3 | 3.3 |
| Benzothiazoles | ||||||||||
| A | 4e | 2-Benzo[d]thiazole | H | H | n/a | n/a | 0.077d | 0.18d | 2 | 1.8 |
| A | 5 | 2-Benzo[d]thiazole | CH3 | H | n/a | n/a | 0.33d | 1.1d | 3 | 2.7 |
| A | 6 | 2-Benzo[d]thiazole | H | Cl | n/a | n/a | 0.046 (0.0067, 2) | 0.055 (0.037, 4) | 1 | 1.9 |
| n/a | 7 | n/a | n/a | n/a | n/a | n/a | >50d | >50d | n/a | 3.5 |
| Triazoles | ||||||||||
| B | 8 | 4-Methoxyphenyl | n/a | n/a | n/a | n/a | 0.13d | 0.31d | 2 | 0.75 |
| B | 9 | 4-Methoxybenzyl | n/a | n/a | n/a | n/a | 0.16 (0.054, 4) | 0.45 (0.12, 4) | 3 | 1.1 |
| B | 10 | 6-Benzo[d]thiazole | n/a | n/a | n/a | n/a | 0.027 (0.0018, 2) | 0.069 (0.017, 4) | 3 | 1.5 |
| B | 11 | 1-(β-d-Galactopyranosyl, 2,3,4,6-tetraacetate) | n/a | n/a | n/a | n/a | 10 (6.2, 4) | >50 (n/a, 3) | >5 | −0.30 |
| B | 12 | CH2CH2CONHPh | n/a | n/a | n/a | n/a | 2.7 (0.084, 2) | 18 (2.0, 2) | 7 | 0.77 |
| Indolyls | ||||||||||
| C | 13 | H | n/a | n/a | CH | CF | 0.10d | 0.17d | 2 | 2.3 |
| C | 14 | H | n/a | n/a | CH | CH | 0.053d | 0.11d | 2 | 2.3 |
| C | 15 | H | n/a | n/a | N | CH | 0.022 (0.0026, 4) | 0.041 (0.021, 4) | 2 | 1.4 |
| C | 16 | CH3 | n/a | n/a | CH | N | 0.026 (0.0065, 4) | 0.044 (0.028, 4) | 2 | 1.8 |
| C | 17 | NHCO2Et | n/a | n/a | CH | CH | 0.050 (0.016, 4) | 0.086 (0.029, 4) | 2 | 2.3 |
| C | 18 | CH2CH2NHAc | n/a | n/a | CH | CH | 0.012 (0.0019, 2) | 0.036 (0.0064, 4) | 3 | 1.9 |
| C | 19 | CH2CH2CO2CH3 | n/a | n/a | CH | CH | 0.16 (0.066, 14) | 0.28 (0.14, 13) | 2 | 2.4 |
| C | 20 | CH2CH2CH2OH | n/a | n/a | CH | CH | 0.024 (0.0017, 2) | 0.051 (0.015, 4) | 2 | 2.1 |
| C | 21 | CH2CH2CO-N-morpholine | n/a | n/a | CH | CH | 0.036 (0.0097, 4) | 0.067 (0.014, 4) | 2 | 1.5 |
| C | 22 | CH2CH2CO-4-(1-methylpiperazine) | n/a | n/a | CH | CH | 0.018 (0.0032, 6) | 0.052 (0.022) | 3 | 1.6 |
| C | 23 | CH2CH2CH2-N-morpholine | n/a | n/a | CH | CH | 0.026 (0.00042, 2) | 0.079 (0.043, 4) | 3 | 2.5 |
| C | 24 | CH2CH2CH2-4-(1-methylpiperazine) | n/a | n/a | CH | CH | 0.016 (0.0063, 4) | 0.050 (0.023, 4) | 3 | 2.6 |
| C | 25 | SO2CH3 | n/a | n/a | CH | CH | 0.024 (0.0017, 4) | 0.039 (0.0072, 4) | 2 | 1.5 |
n/a is not applicable.
The EC50 values are the arithmetic mean of independent determinations with the standard deviations (SD) followed by the number of repeats (n) given in parentheses.
Species selectivity (Spec. select.) is defined as EC50 (MRC5)/EC50 (T. brucei).
Calculated log P values generated using the software package StarDrop by Optibrium.24
Data taken from Konig et al.21
Previously published as PMX 464.