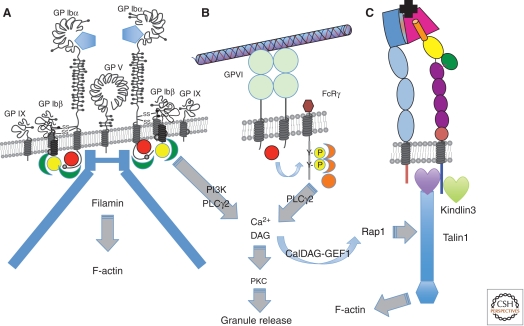
Figure 3.
Platelet receptors for ECM proteins. (A) The GPIb-V-IX complex—the major receptor for von Willebrand factor (VWF), which binds through its A1 VWA domain (blue pentagon) to the LRR repeat region of GPIbα. The receptor is constitutively active and has nine subunits: two copies of GPIbα, each of which associates with two copies of GPIbβ and one of GPIX—the two GPIb-IX heterotetramers are associated with a single copy of GPV. The cytoplasmic domains of GPIbα and GPIbβ bind filamin and a number of signal transduction proteins, including Src (red sphere), 14-3-3 (green), and calmodulin (yellow), and activate PI3-kinase and phospholipase Cγ2. (B) GPVI, a receptor for fibrillar collagens, has two Ig domains and is noncovalently associated with the Fc receptor-γ (FcRγ) chain, which acts as a signal transduction subunit. On ligand binding, a Src family kinase (red sphere) phosphorylates two tyrosine residues in the immunoreceptor tyrosine-based activation motif (ITAM) motif within the FcRγ chain. Syk kinase (orange) binds the phosphorylated ITAM motif and activates phospholipase Cγ2. Ca2+ generated downstream from IP3 released by PLC stimulates the Rap-GEF, CalDAG-GEF1, to activate Rap1, which leads to association of talin-1 with integrins, thereby activating them. Diacylglycerol (DAG) also induces PKC-mediated release of granule contents, including soluble agonists and ECM components. (C) Integrins are activated by the VWF and collagen receptors (or by GPCRs bound by other platelet agonists) through binding of talin-1 and kindlin-3 to their β subunit cytoplasmic domains. Once activated, integrins can bind their ECM protein ligands. Several β1 integrins are expressed on platelets at a few thousand copies per platelet, most notably α2β1 and α6β1, receptors for collagens and laminins, respectively, but also α5β1, a receptor for fibronectin and fibrillin and αvβ3, which binds many ECM proteins (see text). The major platelet integrin, αIIbβ3, is present at 60–80,000 copies per platelet and is the major receptor mediating platelet aggregation through its binding to fibrinogen (and also to fibronectin, VWF, and several other ECM proteins—see text). Most integrins bind their ligands (black) through a binding site at the junction between the head domains of the two subunits—a β propellor domain in the α subunit (dark blue) and a VWA domain in the β subunit (red). In contrast, α2β1 binds through an additional VWA domain inserted into the β propellor domain (not shown).