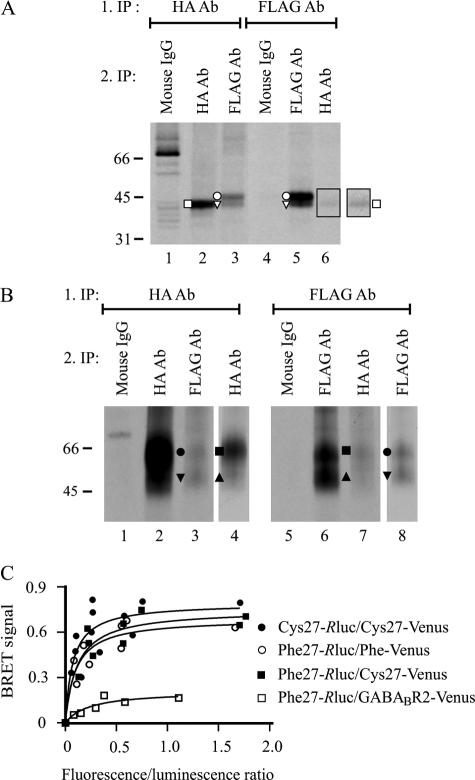
FIGURE 6.
Both precursor and mature receptor forms of hδORCys-27 and hδORPhe-27 variants form homo/heteromers. A and B, co-immunoprecipitation. HEK293i cells constitutively expressing HA-hδORPhe-27 were induced to express Myc-hδORCys-27-FLAG, pulse-labeled with [35S]methionine/cysteine for 40 min, and harvested immediately (A) or chased for 6 h (B). Receptors were purified from cellular lysates by sequential immunoprecipitation, performing the first step in native conditions with the FLAG M2 or HA antibody. Proteins were eluted with SDS-containing buffer, and re-immunoprecipitation was performed from the diluted denatured eluates with the FLAG M2 or HA antibodies or the mouse IgG, as indicated. Only one-eighth and one-fourth of the samples was loaded on lanes 2 and 5 in panel A and on lanes 2 and 6 in panel B, respectively. In panel A, the outlined squared area of lane 6 is shown with enhanced contrast. In panel B, the lanes 4 and 8 represent shorter exposures of lanes 2 and 6, respectively. The precursor and mature receptor forms are indicated with open and closed symbols, respectively, as specified in Fig. 1. The difference in migration of the 2-N-glycan precursors of the two variants (A) is because of the distinct N- and C-terminal tags. C, BRET measurements. HEK293 cells were transiently co-transfected with different amounts of energy donors (hδORCys-27-Rluc, hδORPhe-27-Rluc) and energy acceptors (HA-hδORCys-27-Venus, HA-hδORPhe-27-Venus, HA-GABABR2-Venus) as indicated. After 48 h, BRET signals were measured after the addition of the luciferase substrate coelenterazine H. The results shown are plotted as a function of the ratio of total fluorescence (Venus) and total luminescence (Rluc) and are representative of two independent experiments carried out in duplicate. The curves were fitted using a non-linear regression analysis with a single binding site.