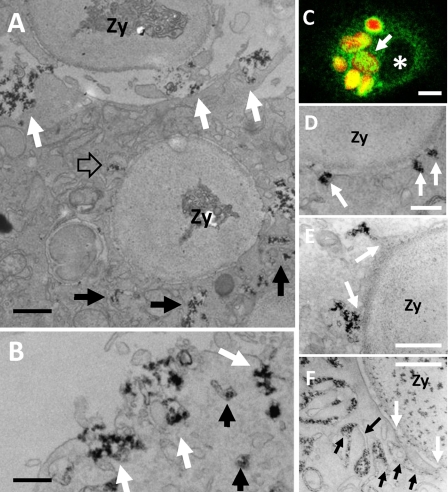
FIGURE 2.
CeCl3 cytochemistry in Ra2 microglia phagocytosing IgG-opsonized zymosan. A–F, LPS-stimulated Ra2 microglia were challenged with IgG-opsonized zymosan particles and either used for immunofluorescence (C) or for CeCl3 cytochemical detection of superoxide production (A, B, and D–F). A shows a cell that has completed uptake of one IgG-opsonized zymosan particle (Zy) and is in the process of phagocytosing another (at top). Note the build up of membrane-bound compartments containing cerium hydroxide reaction product around the phagosome (black arrows), one of which is seen to fuse with the limiting membrane of the phagosome (open arrow). Reaction product is also released at the base of the phagocytic cup by exocytosis of larger vacuolar elements (white arrows). Bar, 1 μm. B, exocytosis of vacuolar elements with cerium hydroxide deposit could also be seen on the general plasma membrane (white arrows). Bar, 500 nm. C shows by immunofluorescence one Ra2 microglial cell that has phagocytosed several IgG-opsonized, Texas Red-conjugated zymosan particles (red), which are all surrounded by an intense patchy immunoreactivity for gp91phox (green). Asterisk denotes nucleus. Bar, 10 μm. D–F, examples of delivery of reaction product to phagosomes containing IgG-opsonized zymosan. D shows direct fusion of 100 nm vesicles with the limiting membrane of the phagosome (arrows), but more commonly cerium hydroxide reaction product was released into nascent phagosomes by fusion with larger vesicles and vacuoles (arrows in E). Some phagosomes were surrounded by lamellar and/or tubular membranes containing the reaction product (black arrows), some of which fused with the limiting membrane of the phagosome (white arrows). Bars, 250 nm in D; 500 nm in E and F.