FIGURE 5.
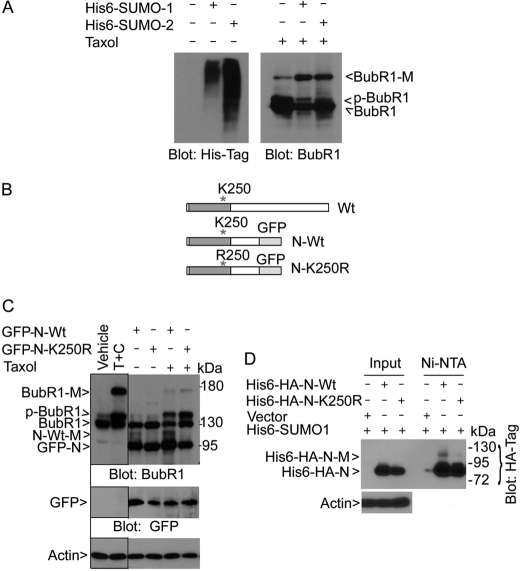
BubR1 K250 is crucial for sumoylation. A, HeLa cells constitutively expressing His6-SUMO-1 or His6-SUMO-2, as well as parental HeLa cells, were collected and equal amounts of cell lysates were blotted with the antibody to the His6 tag. These cells were also treated with taxol for 18 h after which cell lysates were prepared. Equal amounts of cell lysates were blotted for BubR1. B, schematic presentation of BubR1 (Wt) and its N-terminal fragment (610 amino acids) with (N-K250R) or without (N-Wt) K250 replaced with R250. GFP was fused in-frame with BubR1 N-terminal fragment. C, HeLa cells transfected with a plasmid construct expressing GFP-tagged N-terminal fragment of BubR1 (GFP-N-Wt) or its mutant counterpart (GFP-N-K250R) for 24 h followed by taxol treatment for 18 h. Equal amounts of cell lysates were blotted for BubR1, GFP, and β-actin. Arrow N-Wt-M denotes the sumoylated, GFP-tagged N-terminal fragment of BubR1. Arrow GFP-N denotes both the wild-type N-terminal fragment of BubR1 and its mutant counterpart. Vehicle and T+C denote lysate inputs that were derived from cells treated with vehicle or taxol (T) plus caffeine (C) for 18 h, respectively. D, HeLa cells constitutively expressing His6-SUMO-1 were transfected with His6-HA-N-Wt or His6-HA-N-K250R expression plasmids for 48 h. Ectopically expressed proteins were enriched by incubation with Ni-NTA resin and analyzed, along with lysate inputs, by Western blotting using the antibody to HA tag. His6-HA-N-M denotes the sumoylated BubR1 N-terminal fragment.