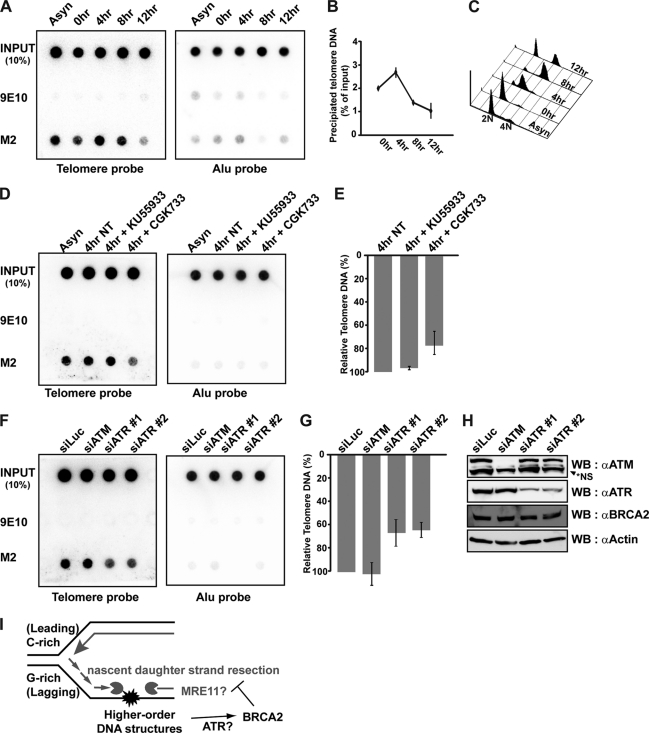
FIGURE 6.
BRCA2 localizes to telomeres during S phase in an ATR-dependent manner. A, HeLa_CFLAP-BRCA2_hTert cells were synchronized at the G1/S boundary by double thymidine block and then released into the cell cycle. Cell lysates were prepared at the indicated time points after thymidine release and subjected to telomere ChIP with anti-FLAG antibody to detect BRCA2 at the telomere (M2). Nonrelated monoclonal 9E10 was employed to control for nonspecific binding. B, percentage of precipitated telomere DNA was measured as a ratio of input signals and marked as dotted graphs at each time point of analysis. C, cell cycle profiles for each time point were measured by FACS analysis. D, HeLa_CFLAP-BRCA2_hTert cells were treated or left alone with 20 μm KU55933 (inhibitor specific for ATM) or 20 μm CGK733 (inhibits ATM and ATR) for 1 and 4 h post-thymidine release. Lysates were also subjected to telomere ChIP with anti-FLAG (M2) to detect BRCA2 bound to telomere. Negative control, 9E10. E and G, bars represent the relative amount of telomere DNA bound to BRCA2 measured by densitometry. The signals were normalized to the input controls. NT, not treated. F, HeLa_CFLAP-BRCA2_hTert cells transfected with indicated siRNAs. Cell lysates were prepared 24 h post-transfection. Telomere ChIP assays were performed with anti-FLAG (M2, BRCA2) and 9E10. Error bars, standard deviation. H, Western blot (WB) analysis to assess the efficiency of siRNAs employed in the assay in F and G. NS, not specific. I, model for the proposed function of BRCA2 in the telomere maintenance. At telomeres, obstacles for elongating DNA polymerase form more frequently at the lagging strands, because G-rich parental strands exposed as single strands between Okazaki fragments can adopt higher order structures such as G-quadruplex. BRCA2 prevents the nascent strand at an aberrant fork from MRE11-dependent uncontrolled resection. In the absence of BRCA2, the resection of daughter strands would be more frequent at lagging strands, resulting in the marked increase of telomere fragility, telomere shortening, and erosion.