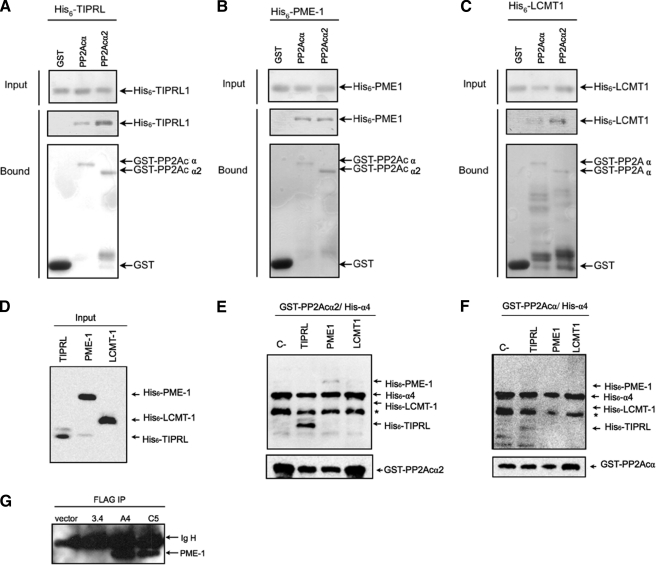
FIGURE 5.
GST pulldown assays using recombinant proteins. A–C, interaction assays of TIPRL, PME-1, and LCMT-1. Fusions of the indicated phosphatase catalytic subunits were co-expressed in E. coli with histidine-tagged TIPRL, PME-1, or LCMT1. GST was used as a negative control. GST fusion proteins were isolated from extracts by binding to glutathione-Sepharose beads. Bound proteins were resolved by SDS-PAGE and detected by immunoblotting with anti-His or anti-GST primary antibodies. Both PP2Ac isoforms interacted specifically with the regulatory proteins tested. D, Western blot analysis using anti-hexahistidine antibody of E. coli extracts expressing histidine-tagged TIPRL, PME-1, and LCMT-1. E and F, interaction assays of the PP2Ac·α4 complexes and TIPRL, PME-1, or LCMT1. GST fusions of the indicated phosphatase catalytic subunits were co-expressed in E. coli with histidine-tagged α4, and the extracts were mixed with the extracts shown in D and incubated for 2 h as 4 °C. Bound proteins were resolved by SDS-PAGE and detected by immunoblotting with anti-hexahistidine antibody (upper panels) or anti GST (lower panels). Only TIPRL interacted with PP2Ac in the presence of α4. The asterisk indicates a proteolysis product of α4. G, Western blot analysis of the FLAG immunoprecipitates shown in Fig. 4C to detect the presence of endogenous PME-1 using anti PME-1 antibody.