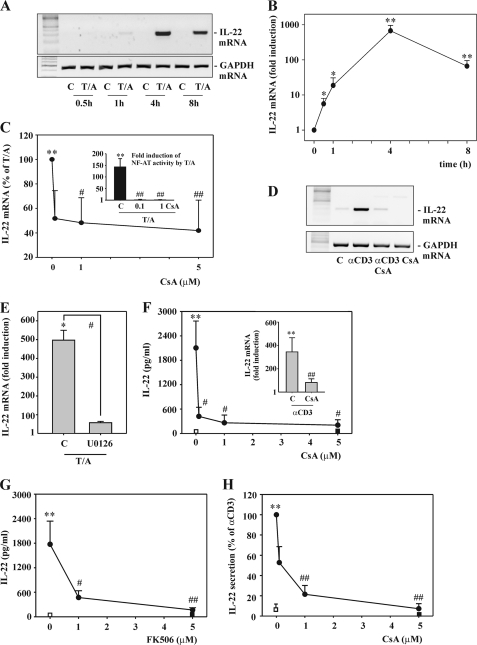
FIGURE 1.
Rapid IL22 gene expression as detected in Jurkat and primary T cells under the influence of T/A or αCD3. A and B, Jurkat T cells were either kept as unstimulated control or stimulated with T (100 ng/ml)/A (10 μm). After the indicated time periods, IL-22 mRNA was assessed by standard (A) or real time PCR (B). A, one representative of three independently performed experiments is shown. B, IL-22 mRNA was normalized to that of GAPDH and is shown as mean fold-induction compared with unstimulated control (at the respective time point) ± S.D. (n = 3); *, p < 0.05, **, p < 0.01 compared with unstimulated control of the respective time point; raw data were analyzed by Student's t test. C, Jurkat T cells were either kept as unstimulated control or stimulated with T (100 ng/ml)/A (10 μm) for 4 h. Where specified, cells had been preincubated for 1 h with the indicated concentrations of CsA before T/A addition. After 5 h, IL-22 mRNA was determined by real time PCR analysis. IL-22 mRNA was normalized to that of GAPDH and is shown as percentage of induction by T/A alone ± S.D. (n = 11); **, p < 0.01 compared with unstimulated control; #, p < 0.05; ##, p < 0.01 compared with induction by T/A alone; raw data were analyzed by one-way ANOVA with post hoc Bonferroni correction. Inset, Jurkat T cells were transfected for 5 h with pNFAT-Luc together with Renilla luciferase as described under “Experimental Procedures.” After 15 h of rest, cells were either kept as unstimulated control or stimulated with T (100 ng/ml)/A (10 μm). Where specified, cells had been preincubated for 1 h with 0.1 or 1 μm CsA before T/A addition. After another 8 h, cells were harvested and luciferase reporter assays were performed. Means of luciferase activity are shown as fold-induction compared with unstimulated control ± S.D. (n = 3); **, p < 0.01 compared with unstimulated control; ##, p < 0.01 compared with T/A alone; raw data were analyzed by one-way ANOVA with post hoc Bonferroni correction. A–C, all cultures were adjusted to a final concentration of 0.1% DMSO (vehicle for T/A, CsA). D, Jurkat T cells were either kept as unstimulated control or stimulated with αCD3 (50 μg/ml) for 12 h. Where indicated, cells had been preincubated for 1 h with CsA (5 μm) before αCD3 addition. After 13 h, IL-22 mRNA was assessed by standard PCR analysis. One representative of three independently performed experiments is shown. All cultures were adjusted to a final concentration of 0.01% DMSO (vehicle for CsA). E, Jurkat T cells were either kept as unstimulated control or stimulated with T (100 ng/ml)/A (10 μm) for 4 h. Where indicated, cells had been preincubated for 1 h with U0126 (10 μm) before T/A addition. All cultures were adjusted to a final concentration of 0.3% DMSO (vehicle for T/A, U0126). After 5 h, IL-22 mRNA was assessed by real time PCR analysis. IL-22 mRNA was normalized to that of GAPDH and is shown as mean fold-induction compared with unstimulated control ± S.D. (n = 3); *, p < 0.05 compared with unstimulated control; #, p < 0.05 compared with T/A alone; raw data were analyzed by one-way ANOVA with post hoc Bonferroni correction. F, PBMC were either kept as unstimulated control or stimulated with αCD3 (7 μg/ml) for 24 h. Where specified, cells had been preincubated for 1 h with the indicated concentrations of CsA before αCD3 addition. All cultures were adjusted to a final concentration of 0.01% DMSO (vehicle for CsA). After 25 h, IL-22 release was determined by ELISA. Data are expressed as mean ± S.E. (n = 3); open square denotes unstimulated control, closed square denotes CsA (5 μm) alone; **, p < 0.01 compared with unstimulated control; #, p < 0.05 compared with αCD3 alone; raw data were analyzed by one-way ANOVA with post hoc Bonferroni correction. Inset, PBMC were either kept as unstimulated control or stimulated with αCD3 (7 μg/ml) for 24 h. Where indicated, cells had been preincubated for 1 h with CsA (5 μm) before αCD3 addition. All cultures were adjusted to a final concentration of 0.01% DMSO (vehicle for CsA). After 25 h, IL-22 mRNA was assessed by real time PCR. IL-22 mRNA was normalized to that of GAPDH and is shown as mean fold-induction compared with unstimulated control ± S.E. (n = 4); **, p < 0.01 compared with unstimulated control; ##, p < 0.01 compared with αCD3 alone; raw data were analyzed by one-way ANOVA with post hoc Bonferroni correction. G, PBMC were either kept as unstimulated control or stimulated with αCD3 (7 μg/ml) for 24 h. Where specified, cells had been preincubated for 1 h with the indicated concentrations of FK506 before αCD3 addition. All cultures were adjusted to a final concentration of 0.05% DMSO (vehicle for FK506). After 25 h, IL-22 release was determined by ELISA. Data are expressed as mean ± S.E. (n = 6); open square denotes unstimulated control, closed square denotes FK506 (5 μm) alone; **, p < 0.01 compared with unstimulated control; #, p < 0.05; ##, p < 0.01 compared with αCD3 alone; raw data were analyzed by one-way ANOVA with post hoc Bonferroni correction. H, primary CD3+ T cells were either kept as unstimulated control or stimulated with αCD3 (7 μg/ml) for 24 h. Where specified, cells were preincubated for 1 h with the indicated concentrations of CsA before αCD3 addition. All cultures were adjusted to a final concentration of 0.01% DMSO (vehicle for CsA). After 25 h, IL-22 release was determined by ELISA. Data are expressed as percent of αCD3 stimulation ± S.E. (n = 5–13); open square denotes unstimulated control, closed square denotes CsA (5 μm) alone; **, p < 0.01 compared with unstimulated control; ##, p < 0.01 compared with αCD3 alone. Raw data were analyzed by one-way ANOVA with post hoc Bonferroni correction.