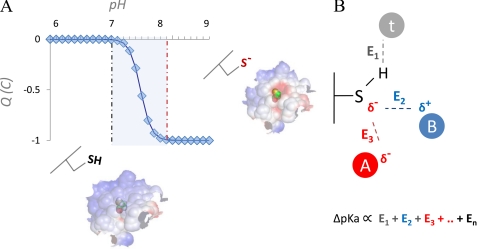
FIGURE 1.
Effect of pH perturbation on net charge of exposed Cys and electrostatic properties of molecular surface. Exposed Cys residues are significantly more polar than buried Cys residues, a consequence of the high degree of Cys polarizability. For many exposed and polar Cys residues, pKa is close to the physiological pH (A, blue, shaded). In such cases, for a typical monoprotic acid in a physiological solution (assuming Henderson-Hasselbalch behavior), sudden negative charge switches can occur in the response to even very limited local pH shifts (A, neutral Cys for lower pH values and anionic Cys for higher pH values; note the steep transition between the two Cys forms in the shaded area). For any Cys in the protein, its interactions with other titratable groups and solvent determine the degree of reactivity of that Cys and influence its pKa. From a computational perspective, a common way to quantify the pKa of a residue is to calculate its deviation (ΔpKa) from the reference pKa value (pKa(REF)) for that amino acid type; ΔpKa is derived by properly accounting for all interactions with the titratable functional group, e.g. E1, E2, … En in B, where interactions with the generic titratable residues A (red circle; indicating an interacting acidic residue), B (blue circle, indicating an interacting basic residue), and t (gray circle; indicating a generic non-charged titratable residue, e.g. Thr) are shown. The ΔpKa then allows the pKa of a residue to be expressed as pKa = pKa(REF) + ΔpKa.