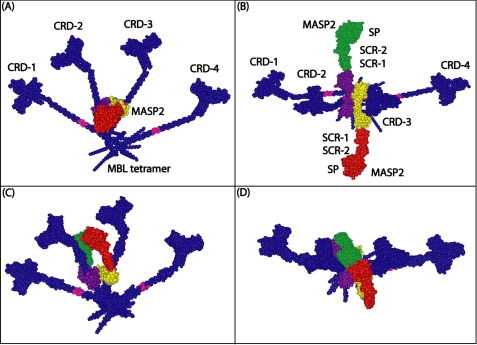
FIGURE 7.
Model of the MASP-2 interaction with the rat MBL tetramer. The best-fit MBL tetramer model is shown in dark blue with the MASP-binding motif Hyp-Gly-Lys-Leu-Gly-Pro highlighted in pink on the collagen triple helix. MASP-2 is constructed from six domains in the order CUB1-EGF-CUB2-SCR-1-SCR-2-SP (CUB, C1r/C1s, Uefg, and bone morphogenetic protein-1; EGF, epidermal growth factor; SCR, short complement regulator; SP, serine protease). The MASP-2 model was created from crystal structures for the CUB1-EGF-CUB2 dimer, with each monomer shown in yellow or purple (PDB code 1NT0) (56). The remaining SCR-1-SCR-2-SP domain pair is shown in red and green (PDB code 1ZJK) (58). A, face-on view of the near-planar MBL tetramer in which the four CRD regions CRD-1 to CRD-4 are visible, and the linear MASP-2 dimer is shown end-on. B, view of A is turned by 90° about the x axis so that the full length of the MASP-2 dimer is seen together with its two serine protease and four short complement regulator domains. C, in an auto-activation model for MASP-2, the two serine protease domains of the MASP-2 dimer are folded back relative to the CUB-EGF domains that are positioned on the triple helical collagen regions. The two serine protease domains form contacts with each other in the plane of the MBL tetramer, as required for an auto-activation mechanism. D, view of C is turned by 90° about the x axis so that the postulated contact between the green and red serine protease domains is seen more clearly.