FIGURE 3.
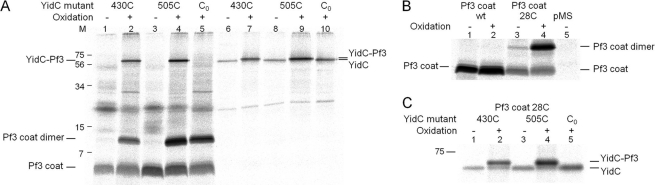
Pf3-YidC disulfide complexes are recognized by antibodies to Pf3 and to YidC. A, coexpression of Pf3-28C and YidC with a single cysteine at residue 430 (lanes 1, 2, 6, and 7) and 505 (lanes 3, 4, 8, and 9) were analyzed as described for Fig. 2, except that in lanes 1, 3, 6, and 8 no copper phenanthroline was added. Lanes 1-5 were immunoprecipitated with an antibody to Pf3. Lanes 6-10 were immunoprecipitated with an antibody to YidC. As a control, the cysteine-less YidC mutant (lanes 5 and 10) was expressed. The Pf3 coat protein was found as a monomer, dimer, and cross-linked with YidC. Molecular weight markers are indicated in kDa at the left margin (lane M). B, E. coli MK6 cells expressing the wild-type Pf3 coat protein (lanes 1 and 2) and Pf3–28C (lanes 3 and 4) were pulse-labeled as described above and treated with copper phenanthroline (lanes 2 and 4). The empty vector is shown as a control (lane 5). C, for better separation, lanes 6-10 of Fig. 3A were applied to a long PAGE.