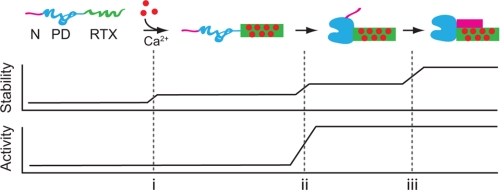
FIGURE 6.
Alkaline protease folding pathway. A putative pathway for the vectoral folding and stabilization of AP is presented. Ca2+ binding to the RTX domain initiates its folding (i). The native RTX domain facilitates the folding and activation of the PD via the 1000-Å2 RTX-PD interface (ii). The folded PD, in turn, facilitates the N-terminal helix-RTX interaction by placing the N-terminal helix in position to bind the folded RTX domain (iii). Stability of AP increases with each domain-domain association. Stabilization of the RTX domain structure occurs upon its interaction with Ca2+, its association with the PD, and its association with the N-terminal helix. The activity of AP is regulated by the association of the PD with the folded, Ca2+-bound RTX domain. The AP domains are colored as follows: N-terminal helix (magenta), PD (blue), and RTX (green) as in Fig. 1A. Folded protein is shown as a solid shape; unfolded protein domains are shown as extended lines.