FIGURE 5.
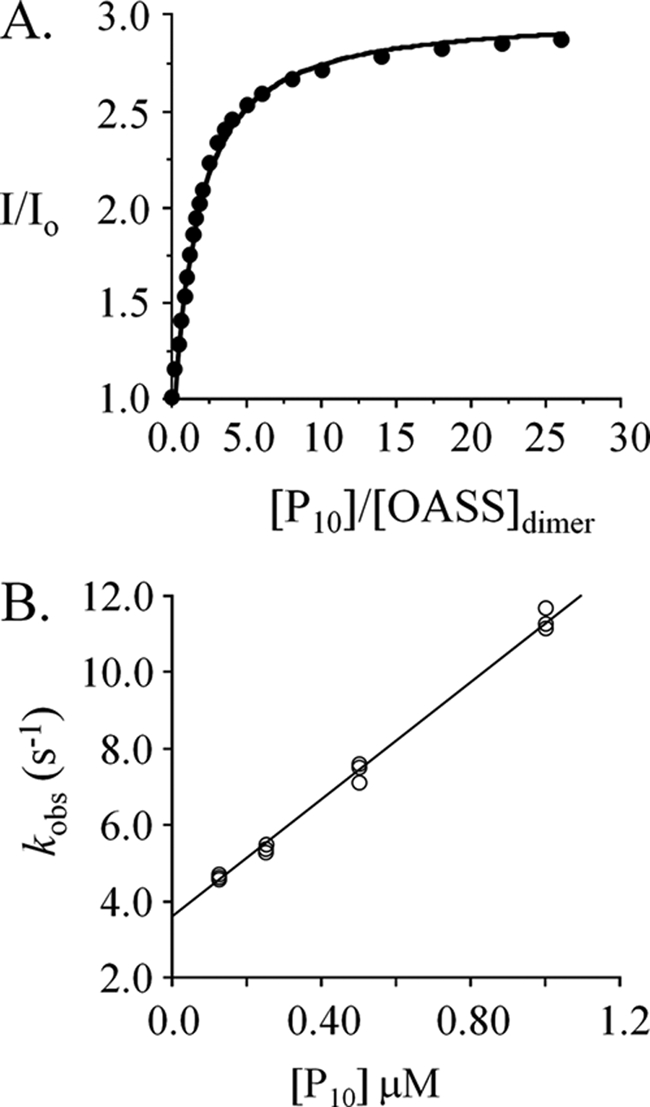
The interaction of peptide (P10) with OASS. A, the dissociation constant. Peptide binding to OASS was monitored via fluorescence changes at 510 nm (λex 414 nm). Titrations were carried out in Hepes/K+ (50 mm, pH 7.0) at 25 ± 2 °C. The concentration of OASSdimer was 0.50 μm. B, rate constants. Binding was initiated by rapidly mixing (1:1) OASSdimer (6.25 nm, final) with P10 (at the concentrations indicated). Solutions were buffered using Hepes/K+ (50 mm, pH 7.0) and equilibrated at 25 ± 2 °C prior to mixing. Reaction progress was monitored using fluorescence (λex 414 nm, λem > 455 nm). kobs values were determined in triplicate: each value was obtained by fitting the average of ten progress curves to a single-exponential decay. kon (7.7 × 106 m−1 s−1) and koff (3.6 s−1) were obtained by fitting the kobs versus [P10] data using the equation: kobs = kon · [pep] + koff.
