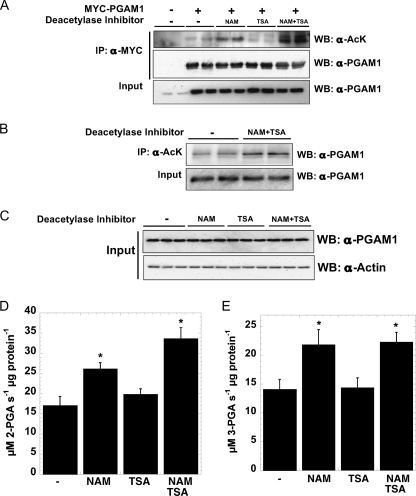
FIGURE 1.
PGAM1 is acetylated in vivo, and acetylation increases PGAM1 activity. A, transiently transfected PGAM1 is acetylated in vivo, when treated with sirtuin-specific inhibitors. MYC-PGAM1 was overexpressed in HEK293 cells and treated overnight with 5 mm NAM, 5 μm TSA, and 5 mm NAM, 5 μm TSA. MYC-PGAM1 was immunoprecipitated (IP) by anti-MYC conjugated to agarose at 4 °C for 4 h. These extracts were resolved by SDS-PAGE and detected by Western blotting (WB) with anti-acetyl-lysine (AcK) and anti-PGAM1 antibodies, respectively. Input refers to 1% of total lysate and was detected with anti-PGAM1 antibody. B, endogenous PGAM1 is acetylated in vivo. Endogenous PGAM1 is immunoprecipitated by anti-acetyl-lysine and protein G from HEK293 cells incubated overnight with 5 mm NAM, 5 μm TSA. These extracts (Input = 1% total cell lysate) were resolved by SDS-PAGE and detected by Western blotting with anti-PGAM1 antibody. C–E, soluble extracts of HEK293 cells incubated overnight with 5 mm NAM, 5 μm TSA, and 5 mm NAM, 5 μm TSA were resolved by SDS-PAGE and detected by Western blotting with anti-PGAM1 and anti-actin antibodies (C), respectively; activity assays measure PGAM1 activity. Input refers to 1% total cellular lysate. Forward PGAM reaction (D), coupled to Enolase, pyruvate kinase, and lactate dehydrogenase, utilized saturating 3-phosphoglycerate measuring absorbance at 340 nm. Reverse PGAM reaction (E), coupled to phosphoglycerate kinase and GAPDH, utilized saturating 3-phosphoglycerate measuring absorbance at 340 nm. Error bars represent S.E. (n = 3); *, p < 0.05 compared with untreated cells.