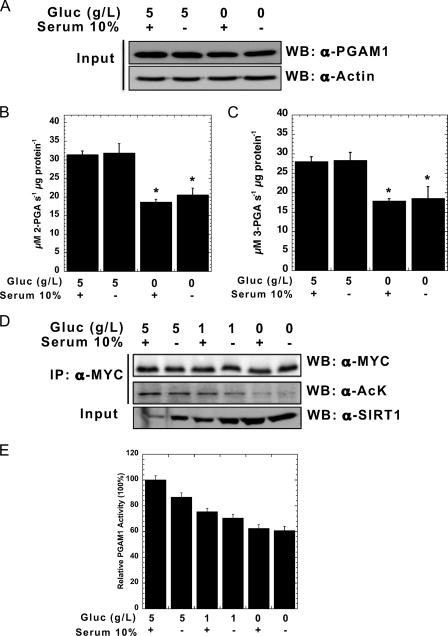
FIGURE 4.
High glucose increases PGAM1 acetylation and activity in vivo. A, HEK293 cells were treated by 5 g/liter glucose (Gluc) ± FBS and 0 g/liter glucose ± FBS for 6 h. Endogenous PGAM1 activity assays were measured at 340 nm for both forward (B) and reverse reactions (C). Cell lysates were analyzed by SDS-PAGE and detected through Western blot (WB) with anti-PGAM1 and anti-actin antibodies. Input refers to 1% total cellular extract (A). D, MYC-PGAM1 was overexpressed in HEK293 cells following incubation for 6 h with 5 g/liter glucose ± FBS, 1 g/liter glucose ± FBS, and 0 g/liter glucose ± FBS. Cell lysates were resolved by SDS-PAGE, and Western blotting was performed with anti-SIRT1, anti-MYC, and anti-acetylated lysine antibodies. E, MYC-PGAM1 was immunoprecipitated (IP) with anti-MYC conjugated to agarose, and PGAM1 activity assay was measured. PGAM1 protein levels were normalized using quantification of the anti-MYC Western as in D. Input refers to 1% cellular extract. Error bars represent S.E. (n = 3); *, p < 0.05 compared with 5 g/liter glucose with serum.