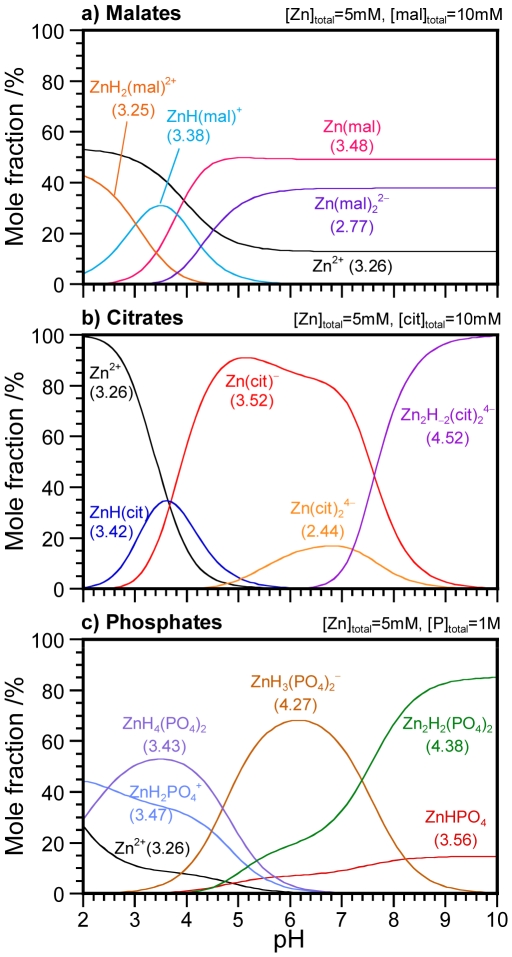
Figure 2. Mole fractions of Zn species as functions of pH at 298 K.
Mole fractions of Zn species were calculated by using formation constants and acid dissociation constants reported in the literature [18], [20]–[22]. The ln β values at 298 K are shown in parentheses. a) Mole fractions of Zn species in malate solutions. Total concentrations of Zn and malate are 5 mM and 10 mM, respectively. b) Mole fractions of Zn species in citrate solutions. Total concentrations of Zn and citrate are 5 mM and 10 mM, respectively. c) Mole fractions of Zn species in orthophosphate solutions. Total concentrations of Zn and phosphate are 5 mM and 1 M, respectively.