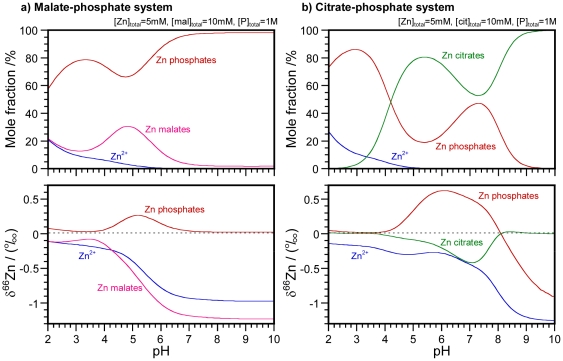
Figure 3. Isotope fractionation δ66Zn of hydrated Zn2+, and aqueous citrates, malates, and phosphates at 298 K.
Mole fractions of Zn species were calculated by using formation constants and acid dissociation constants reported in the literature [18], [20]–[22]. Isotope fractionation δ66Zn (‰) compared with the bulk solution (averaged δ66Zn in the whole solution, δ66Zn = 0) is shown together as a dotted line. The calculation procedure of δ66Zn is from [9]. a) Malate-phosphate system. Total concentrations of Zn, malate, and phosphate are 5 mM, 10 mM, and 1 M, respectively. b) Citrate-phosphate system. Total concentrations of Zn, citrate, and phosphate are 5 mM, 10 mM, and 1 M, respectively.