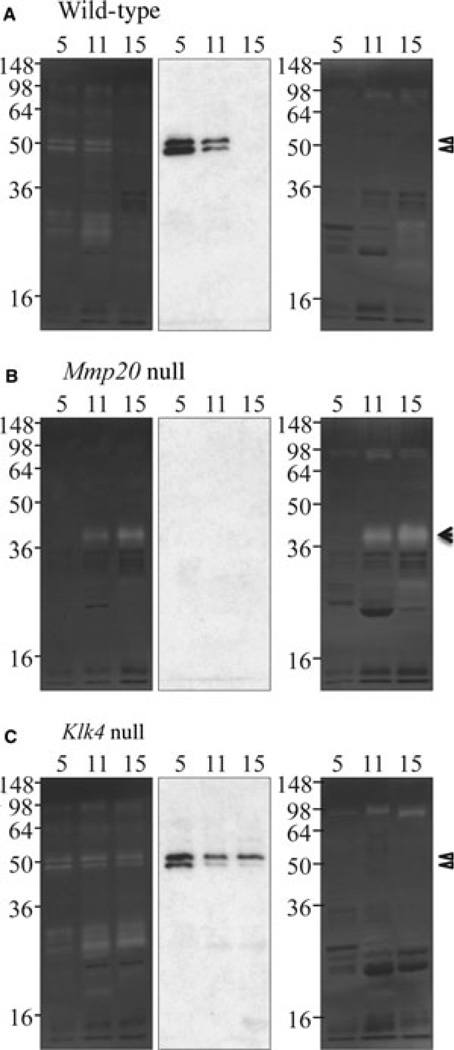
Fig. 4.
Matrix metalloproteinase 20 (MMP20) in kallikrein-related peptidase 4 (Klk4) null mice. Matrix metalloproteinase 20 is easily detected on casein zymograms and western blots. Enamel proteins from days 5 (2 µg), 11, and 15 were analyzed on casein zymograms with calcium (left) or with EDTA and phenanthroline (MMP inhibitors) (right), and by western blotting (center) using an MMP20 C-terminal antibody. Matrix metalloproteinase 20 was not detected in the Mmp20 null mice. Day 5: MMP20 was detected in wild-type and Klk4 null mice as a doublet near 50 kDa, but not on zymograms incubated with MMP inhibitors. The MMP20 catalytic domain was responsible for a group of caseinolytic bands around 30 kDa. Day 11: MMP20 was detected in wild-type and Klk4 null mice. An unidentified 40-kDa protease was evident in the Mmp20 null mice and was active in the presence of MMP inhibitors. Day 15: MMP20 was only detected in the Klk4 null mice. The 40-kDa protease was also detected. Triangles indicate MMP20 bands and the arrowhead indicates an unidentified 40-kDa protease.