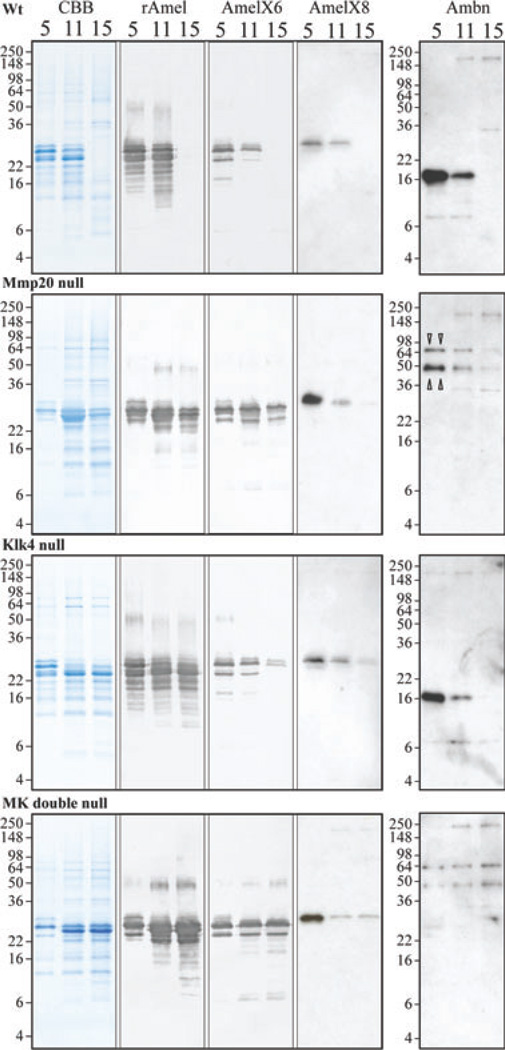
Fig. 5.
SDS-PAGE and western blot analyses of amelogenin and ameloblastin at days 5, 11, and 15 from wild-type (Wt), matrix metalloproteinase 20 (Mmp20) null, kallikrein-related peptidase 4 (Klk4) null, and Mmp20/Klk4 (MK) double-null mice. Panels show (from left to right): Coomassie Brilliant Blue-stained SDS-polyacrylamide gels; western blots with rM179 antibody (rAmel); western blots with amelogenin C-terminal antibodies specific for the exon 6-encoded sequence (AmelX6) and for the exon 8-encoded sequence (AmelX8); and western blots with the ameloblastin (Ambn) antibody for the N-terminal domain. All day-5 lanes show 2-µg loads. Day-11 and day-15 lanes from each genotype show the same percentage of protein per tooth (see Table 1). Bands on western blots using amelogenin C-terminal antibodies are uncleaved amelogenin isoforms. Note: all amelogenin-positive bands in the day-5 MMP20 null and MK double-null mice co-migrate with intact amelogenin and the wild-type and Klk4 null samples are identical at day 5 (secretory stage). Two bands at 65 and 50 kDa in Mmp20 null and MK double-null mice are likely to be uncleaved ameloblastin (triangle pointers). All positive bands of ~200 kDa are immunoglobulins that were also detected in controls using only the secondary antibody (data not shown).