Figure 6. ANXA2 is a substrate of the Trx system.
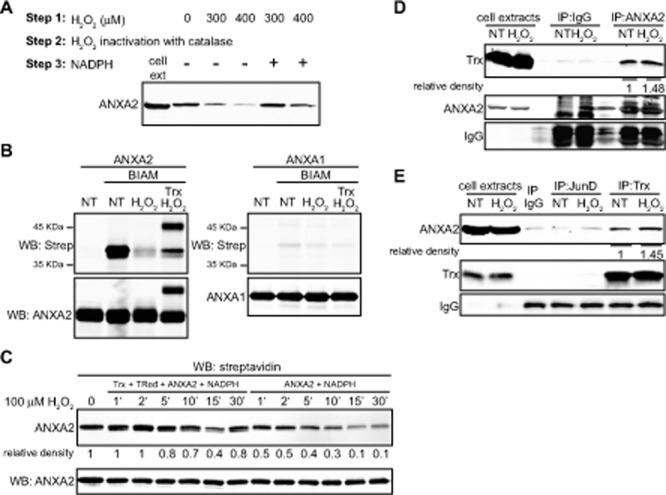
(A) TIME cell extracts were treated with the indicated amounts of H2O2, incubated with catalase after what mock or 500 μM of NADPH was added, as indicated. Lysates were incubated with 10 μM BIAM and labeled proteins were purified with streptavidin beads. Cell extracts of non treated cells (lane 1) and streptavidin purified samples were subjected to SDS-PAGE followed by western blotting for ANXA2. (B) 2.5 μM ANXA2 or ANXA1 were incubated in the absence or presence of 100 μM H2O2 with or without 20 μM of Trx as indicated. Proteins were incubated in the absence or presence of 20 μM BIAM, subjected to SDS-PAGE under non-reducing conditions, followed by western blot analysis with the antibodies indicated. (C) 2 μM of human recombinant ANXA2 were incubated with 0.15 μM Trx reductase, 0.5 μM Trx, 500 μM NADPH (left panel) or with NADPH alone (right panel). 100 μM of H2O2 was added and samples were collected at the times indicated, after what 10 μg/ml of catalase was added. Samples were labeled with 20 μM BIAM and subjected to SDS-PAGE followed by western blotting with a streptavidin probe. (D) A549 cells were either non-treated (NT) or treated with 1 mM H2O2 for 1 hour. Cells were lysed and immunoprecipitated with IgG, anti-JunD or anti-Trx antibodies as indicated; (E) A549 cells were either non-treated (NT) or treated with 1 mM H2O2 for 1 hour. Cells were lysed and immunoprecipitated with either IgG or anti-ANXA2 antibodies as indicated (E) A549 cells were either non-treated (NT) or treated with 1 mM H2O2 for 1 hour. Cells were lysed and immunoprecipitated with IgG, anti-JunD or anti-Trx antibodies as indicated. (D-E) Immunoprecipitates and 20 μg of each cell extract were subjected to SDS-PAGE followed by western blotting with the antibodies indicated. Protein band quantification was done using the Licor Odyssey software.
