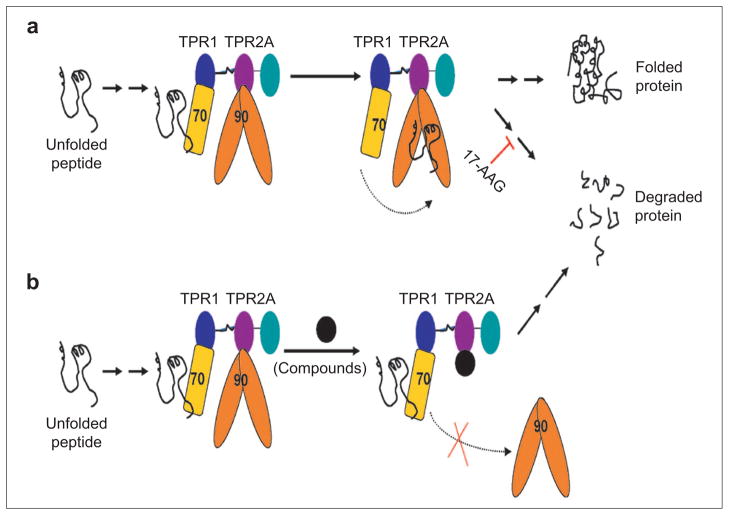
Figure 1.
Schematic illustration of Hsp90-mediated protein folding and proposed mechanism of identified compounds. a) For Hsp90-dependent proteins, the newly synthesized polypeptide chain (black line) is chaperoned through the initial steps of folding by Hsp70 (yellow rectangle). Hsp70 and Hsp90 (orange dimer) are brought together in a complex, via their interactions with the TPR1 (blue) and TPR2A (purple) domains of HOP, respectively. Partially folded polypeptide is passed from Hsp70 to Hsp90, where the final steps of folding occur. If Hsp90 activity is inhibited, for example, by 17-AAG, the Hsp90-dependent protein, e.g., HER2, dissociates from the multichaperone complexes, does not complete its folding, and is targeted for degradation by the proteasome. b) Proposed mode of action of molecules that inhibit the Hsp90–HOP interaction. The first, Hsp70-dependent step of folding will initiate as normal. The inhibitory molecule will prevent the interaction of Hsp90 with HOP. Thus Hsp90 cannot form the HOP-mediated complex with Hsp70 and consequently cannot receive the partially folded substrate. The incompletely folded protein is targeted for degradation by the proteasome.