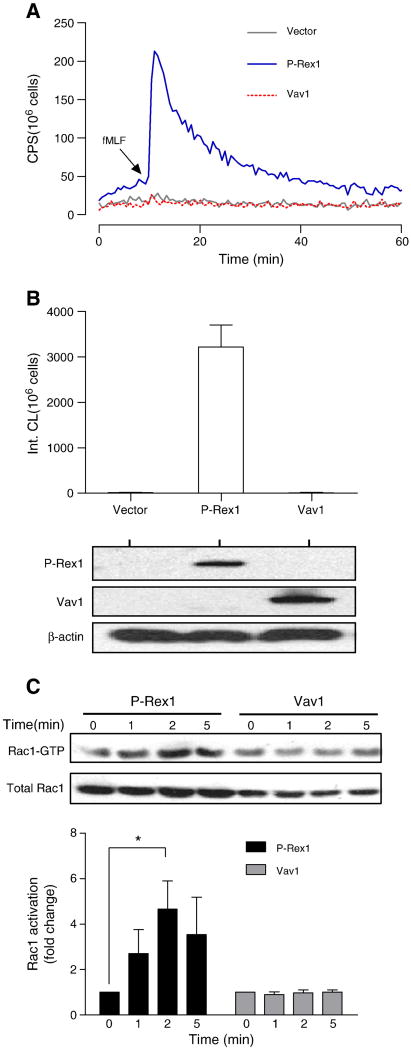
Fig. 1.
P-Rex1-dependent reconstitution of fMLF-induced NADPH oxidase activation. COSphox cells were transiently transfected with expression constructs for FPR and either P-Rex1 or Vav1. Superoxide production assays were conducted 24 h after transfection. (A) fMLF (1 μM)-induced superoxide production shown as changes in isoluminol-enhanced chemiluminescence (CL). CPS, counts per second. (B) Quantification of superoxide production based on integrated chemiluminescence (Int. CL) in the first 20 min after stimulation, shown as mean ± SEM from three experiments. The expression level of P-Rex1 and Vav1 was determined with Western blotting using antibodies against AU5 (for AU5-tagged P-Rex1) and FLAG (for FLAG-tagged Vav1). (C) Activation of endogenous Rac1 in COSphox cells after fMLF (1 μM) stimulation for the indicated time. The level of activated Rac1 (Rac1-GTP) was determined using RBD-GST pull-down assay. Total Rac1 was determined with Western blotting using an anti-Rac1 Ab. Densitometric analysis was performed to determine relative activation of Rac1 (Rac1-GTP pulled down, fold change compared to sample at time 0 in each group). Data were normalized against the total Rac1 expression level in 3 independent experiments, and are shown as mean± SEM. * P<0.05. Panel (A) of this figure is colored in the online version.