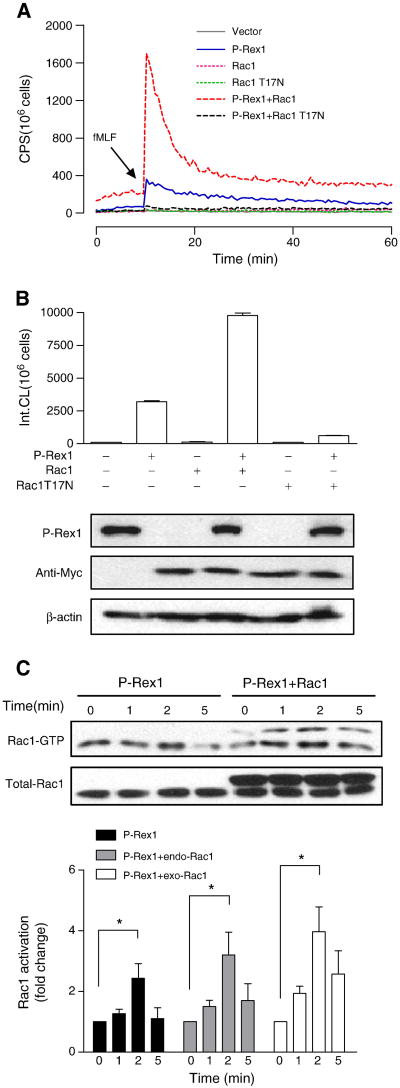
Fig. 3.
P-Rex1-mediated superoxide generation is Rac1-dependent. COSphox cells were cotransfected to express FPR, P-Rex1 and either the WT or T17N mutant of Rac1, both tagged with Myc. (A) fMLF-induced superoxide generation showing maximal oxidant production in the presence of both P-Rex1 and WT Rac1 (compared to P-Rex1 without exogenous Rac1, solid line). Co-transfection of Rac1 T17N ablated the fMLF-induced superoxide generation. (B) Quantification of data in (A), based on isoluminol-enhanced chemiluminescence in the first 20 min after fMLF stimulation. The expressed P-Rex1 and Rac1 constructs were detected with Western blotting using anti-AU5 (for the AU5-tagged P-Rex1) and anti-Myc (for the Myc-tagged WT and T17N mutant of Rac1). β-actin (untagged) was also detected by a monoclonal antibody against β-actin, and was used as a loading control. (C) fMLF-induced Rac1 activation based on RBD-GFP pull-down assay. The Rac1 recovered was then detected using an anti-Rac1 antibody in Western blotting. The upper bands in the blot (right four lanes) represent the Myc-tagged exogenous (exo) Rac1, and the lower bands are endogenous (endo) Rac1 in COSphox cells. Densitometric analysis was performed and relative level of Rac-GTP is shown after normalization against the respective endogenous and exogenous total Rac1. Data are shown as mean±SEM based on three independent experiments. * P<0.05. Panel (A) is shown in color in the online version.