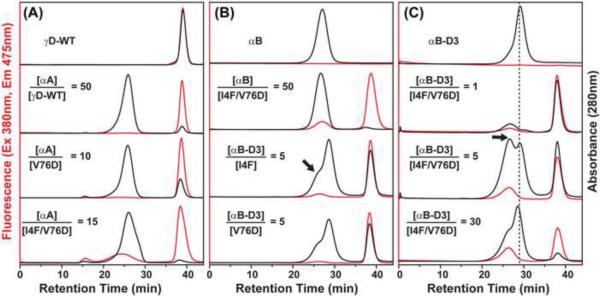
Figure 5.
Analysis of γD-crystallin mutant binding to α-crystallin by size exclusion chromatography (SEC) detected by in-line absorbance at 280 nm and fluorescence at 475 nm. (A) Samples of αA-crystallin and γD-crystallin were incubated at the indicated molar ratios and injected on a Superose 6 column. The binding of γD-crystallin I4F/V76D is manifested by a fluorescence peak with retention time similar to that of αA-crystallin. (B) Comparative SEC analysis of binding to αB-crystallin and αB-D3. A 50-fold molar excess of the former is required for detectable binding of the double mutant while αB-D3 binds the single mutants. The y axes were scaled to show details of the complex peak. (C) The complex between αB-D3 and γD-crystallin is detected as a distinct peak, indicated by the arrow, migrating at a larger mass than αB-D3 alone.