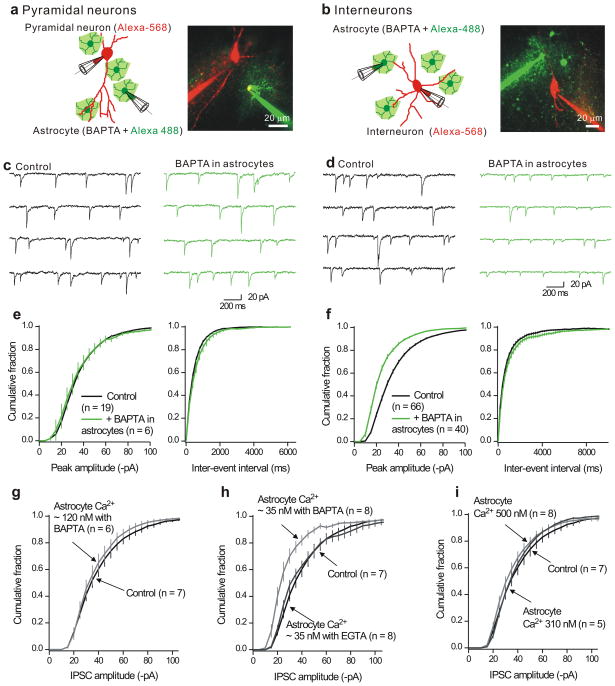
Figure 4. Buffering astrocyte intracellular Ca2+ levels decreases mIPSC amplitudes in interneurons, but not pyramidal neurons in hippocampal slices.
a. The left cartoon shows the protocol whereas the image shows a representative confocal image. The astrocytes were loaded with Alexa-488 (100 μM) whereas the neurons were loaded with Alexa-568 (100 μM). Recordings were made from neurons and astrocytes that were no more than 100 μm apart. In this approach, we recorded from a population of neurons, determined mIPSC parameters and then record from a second population in which the astrocyte nearby had also been patched. b. As in a, but for dual recordings from astrocytes and interneurons in the stratum radiatum region. c. Representative mIPSCs recorded from CA1 pyramidal neurons under controls settings, and also for pyramidal neurons located near astrocytes that were dialyzed with 13 mM BAPTA (>20 min). d. As in c, but for whole-cell recording from interneurons. e. Cumulative probability plots of pyramidal neuron mIPSC amplitudes and inter-event intervals from control neurons and those located near astrocytes dialyzed with BAPTA. f. As in e, but for mIPSCs recorded from interneurons. g–h. Average cumulative probability plots for interneurons located near astrocytes dialyzed with intracellular solutions to clamp the bulk concentration of Ca2+ ions to known levels using either the fast chelator BAPTA, or the slower chelator EGTA. No significant changes were observed for the inter-event interval distributions (Supplementary Fig. 11). In this and all subsequent figures, the vertical lines on the cumulative probability plots represent the standard error of the mean (s.e.m).