Abstract
Potential mechanistic mediators of Darwinian fitness, such as stress hormones or sex hormones, have been the focus of many studies. An inverse relationship between fitness and stress or sex hormone concentrations has been widely assumed, although empirical evidence is scarce. Feathers gradually accumulate hormones during their growth and provide a novel way to measure hormone concentrations integrated over time. Using liquid chromatography–tandem mass spectrometry, we measured testosterone, corticosterone and cortisol in the feathers of house sparrows (Passer domesticus) in a wild population which is the subject of a long-term study. Although corticosterone is considered the dominant avian glucocorticoid, we unambiguously identified cortisol in feathers. In addition, we found that feathers grown during the post-nuptial moult in autumn contained testosterone, corticosterone and cortisol levels that were significantly higher in birds that subsequently died over the following winter than in birds that survived. Thus, feather steroids are candidate prospective biomarkers to predict the future survival of individuals in the wild.
Keywords: corticosteroid, glucocorticoid, mass spectrometry, mortality, Passer domesticus, LC–MS/MS, stress
1. Introduction
Steroid hormones function as mediators of essential metabolic and energy-allocation processes. However, when elevated systemically, steroids can compromise fitness. For example, the glucocorticoids (GCs), cortisol and corticosterone, mobilize energy stores in response to a crisis. However, chronic GC elevation is associated with physiological costs that include heart problems, ulcers, immunosuppression, neuronal damage and decreased survival [1–5]. In several vertebrate species, stress-induced corticosterone levels are associated with survival, either positively [6] or, more often, negatively [7–9].
Testosterone (T) is involved in numerous physiological processes, including neuronal growth and function, muscle and bone development, immune function, feather growth and spermatogenesis in males [10]. High circulating T levels also have major costs, such as reduced fat stores, interference with pair bonds and parental care, and increased injury owing to an increase in activity and aggression [11,12]. T-implanted dark-eyed junco (Junco hyemalis) males display more, lose winter fat deposits more quickly and suffer higher mortality rates than controls [13]. In humans, castrated men with low plasma T levels have lower metabolic rates and live longer [14]. This trade-off between growth, development, reproduction and survival is central to life-history theory.
Feathers are an emerging matrix for endogenous hormone analysis [15]. Obtaining blood samples from wildlife is challenging, especially for steroid hormone analysis, because levels can change within a few minutes (approx. 3 min for GCs to increase [16]). One advantage of using feathers is that they are unaffected by the momentary stress of capture. They reflect steroid levels at the time of growth, thus providing a long-term integrated measure over a specific time period (days to weeks) [15]. Storage does not necessitate special conditions or equipment (i.e. dry envelope, room temperature) and samples need not be from live birds.
In order to assess the fitness correlates of GCs and T, we used flank feathers from house sparrows (Passer domesticus) that were part of a long-term study [17,18]. House sparrows moult only once a year, during the autumn, which is several months after the breeding season and precedes winter stressors. The house sparrow is a well-studied species, from its behavioural ecology to its endocrinology [19–24]. Given the costs of high circulating GCs and T, we hypothesized that higher concentrations of GCs and T in feathers would be associated with higher mortality rates over the subsequent winter.
2. Material and methods
(a). Study species and sample collection
Since 2000, there has been a continuous and systematic investigation of the house sparrow population on Lundy Island, which is located off the coast of southwest England (51°10′ N, 4°40′ W). Adults were captured several times throughout the breeding and non-breeding seasons in 2004–2005 for morphological measurements, taken according to Svensson [25]. Tarsus length (in millimetres) was used as a proxy for body size. Sex and age at feather sampling (1–5 years) were known from demographic data. At this field site, house sparrows moult between late August and early November. After the moult, and following routine morphological measurements, approximately five feathers were gently removed from each side of the flanks before releasing the birds. Flank feathers were chosen to avoid damage to flight feathers, and distributed sampling was used to minimize potential thermoregulatory effects of feather-sampling [26]. Feathers were stored in dry zip-lock bags at room temperature until analysis. House sparrows are highly philopatric, and males typically nest close to their nest from the previous year [19]. Year-to-year resighting probabilities of surviving individuals in the population were close to 99 per cent during our study period [17]. Low levels of migration to and from this island also contribute to coding individual status as ‘alive’ or ‘dead’ without ambiguity [27]. All individuals had their survivorship directly assessed in the breeding season (April–August) following the collection of their feather sample.
(b). Steroid analysis by liquid chromatography–tandem mass spectrometry
We measured cortisol, corticosterone and T using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). LC-MS/MS quantitation eliminates concerns about non-specificity of antibody-based detection methods [28]. LC-MS/MS also allows multiple steroids to be quantified from the same sample in a single run [29]. Cortisol and corticosterone were measured in all samples (n = 61), while T was measured in feathers from a one-year subset (2004–2005) of samples (n = 35).
We pulverized 21–84 mg of feathers (n = 61 subjects) to a fine powder using a ball mill at 25 Hz for 2 min (MM-200; Retsch, Germany). Steroids were extracted overnight into 1 ml of Optima-grade methanol (Fisher Scientific, Fair Lawn, NJ) containing a spike of deuterated cortisol, corticosterone and T (cortisol-9,11,12,12-d4, corticosterone-2,2,4,6,6,17α,21,21-d8, testosterone-1,2-d2; from C/D/N Isotopes Inc., Pointe-Claire, QC, Canada) under gentle shaking and the supernatant was collected. A second extraction was performed on the powdered feather samples using a 30 s vortex with 1 ml diethyl ether (Sigma-Aldrich, St Louis, MO) followed by centrifugation. The methanol and the diethyl ether extracts were combined and evaporated to dryness under a stream of high-purity nitrogen at 45°C using a sample concentrator (Techne Inc., Burlington, NJ).
Samples were resuspended in 5 : 95 methanol : water (1 ml) and subjected to solid-phase extraction (SPE) using Bond Elut C18 SPE cartridges (1 ml, 100 mg sorbent, end-capped; Agilent Technologies, Santa Clara, CA). SPE for both feather and plasma samples was performed via an automated SPE system (Gilson, GX-274 ASPEC, Middleton, WI). After sample-loading, samples were washed twice using 1 ml of de-ionized water. Steroids were eluted from the cartridges using 1 ml of Optima-grade methanol. Standard curves containing known concentrations of steroid standards in water were also spiked with the deuterated steroid mix, carried through sample preparation (SPE) and analysed within the same analytical run.
Cortisol, corticosterone and T were quantified in a single analytical run. Eluates were dried under a stream of nitrogen at 45°C and then resuspended in 80 µl of 50 : 50 methanol : water (Optima grade). Finally, 40 µl of the resuspended sample was injected onto a 100 × 3.00 mm, 2.6 µm Kinetex C18 high-performance liquid chromatography column (Phenomenex, Torrance, CA) using an Agilent 1200 SL LC system with a thermostated autosampler set at 4°C (Agilent Technologies). The chromatographic separation was performed by a gradient elution (15 min) at a flow rate of 0.55 ml min−1 using water (mobile phase A) and methanol (mobile phase B). The separation was carried out at 45°C, and the total run time was 15 min per sample. The LC system was coupled to a QTRAP 5500 tandem mass spectrometer (AB SCIEX, Concord, ON, Canada) fitted with an atmospheric pressure chemical ionization (APCI) source. The nebulizer current was set at 5 µA with a source temperature of 500°C. Nitrogen was produced by a high-purity nitrogen generator (Parker Balston, Haverhill, MA) and was used as the curtain, drying and collision gases.
Analyst software v. 1.5 (AB SCIEX) was employed for data acquisition, peak area integration and calculation of the area ratio relative to the within-sample deuterated standard. Limits of quantitation (LOQ) for our system, based on the concentrations that produced a signal-to-noise (S/N) ratio of ≥10, were 0.05 ng ml−1 for each of corticosterone and cortisol, and 0.1 ng ml−1 for T (equivalent to 2 and 4 pg per analysis, respectively). Data were corrected for feather mass to yield a result quantified as nanogram of steroid per gram of feather.
(c). Statistical analysis
Because many of the feather samples had non-detectable levels of GCs (i.e. below the limit of detection and a zero value in our dataset), we used correlation by randomization to test for associations between steroids. We used nominal logistic regression to test the effect of sex, year, tarsus length, steroid level and their interactions (independent variables) on the probability of survival (dependent variable). Sparrow over-winter survival in this analysis was binary: alive or dead. The overall significance of each model was evaluated by the contribution of the difference between the full model and the reduced model (only the intercept). The contribution of each effect in a model was evaluated using the likelihood-ratio test. We used the Akaike information criterion (AIC) to rank models [30,31]. This approach weighs models by the amount of the variance explained and model complexity (i.e. number of model parameters; K). When n/K < 40, the AIC values were corrected for small sample size (AICc) using the equation of Burnham & Anderson [30]. Level of support for an AICc value was evaluated by ΔAICc (i.e. AICc = AICmodel i − AICmin). Models with ΔAICc values of 0–2 are equally likely, whereas those with ΔAICc > 2 are not supported [30]. All calculations were done in JMP (v. 9.0.1; SAS Institute Inc., Cary, NC).
3. Results
(a). Feather glucocorticoid and testosterone concentrations
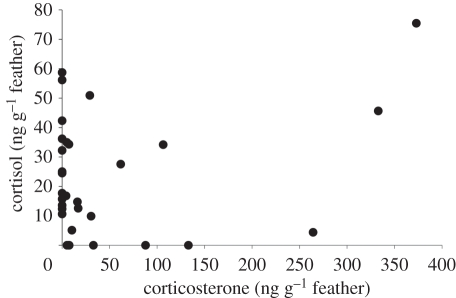
Of the 61 feather samples, 50 samples were above the LOQ for at least one GC. In those 50 samples, there was no difference between corticosterone and cortisol levels (t-test by randomizations: t98 = 1.4, p = 0.18). However, only 16 samples (33%) had detectable levels of corticosterone (range: 4.1–372.9 ng g−1), 25 samples (41%) had detectable levels of cortisol (range: 4.4–75.5 ng g−1) and 13 samples had levels of both GCs above the LOQ. Feather corticosterone and cortisol levels did not differ between the sexes (nmales = 31, nfemales = 19; t-test by randomizations: t48 = 1.1, p = 0.32 and t48 = 0.5, p = 0.64, respectively). Corticosterone and cortisol concentrations in the feathers were significantly correlated (correlation by randomizations: r = 0.40, n = 50, p < 0.01; figure 1), with the variance in corticosterone accounting for 16 per cent of the variance in cortisol. Within the 13 samples with both GCs above the LOQ, cortisol and corticosterone concentrations did not differ (t-test by randomizations: t24 = 1.8, p = 0.11), but the association was not significant (correlation by randomizations: r = 0.51, p = 0.07), probably owing to the limited power.
Figure 1.
In feathers, concentrations of corticosterone and cortisol as measured by LC-MS/MS were correlated within individual samples.
T was detectable in 34 of 35 feather samples. Feather T levels did not differ between the sexes (nmales = 20, nfemales = 15; t-test by randomizations: t33 = 0.1, p = 0.90). Feather T concentrations were associated with feather cortisol concentrations (correlation by randomizations: r = 0.35, n=35, p < 0.05), but not with feather corticosterone concentrations (correlation by randomizations: r = −0.13, n = 35, p = 0.44).
(b). Survival models
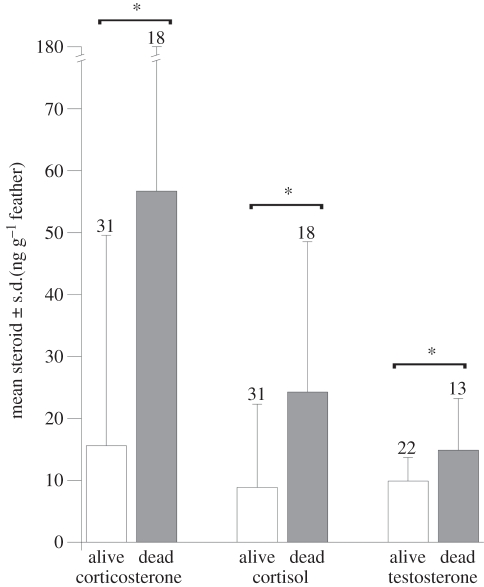
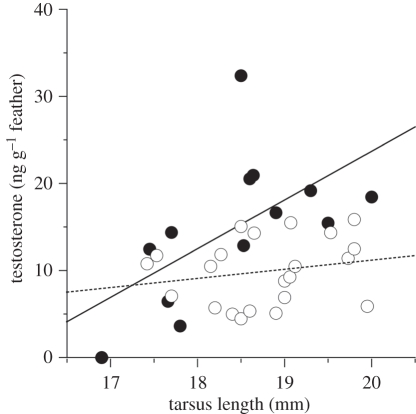
We detected lower concentrations of all the hormones tested in individuals that survived the winter (figures 2 and 3). Non-breeding feather corticosterone levels were significantly lower in birds that survived, relative to those that subsequently died, but only when a year term was included in the model (model 2 in table 1; figure 2). The interaction between year and corticosterone was not significant, and the model with the interaction term was not better than the models lacking it; thus, we left the interaction term out of the models tested (model AICc = 56.9; interaction term likelihood ratio 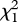 = 3.6, p = 0.06). Individuals that survived also had significantly lower feather cortisol concentrations in all models tested, regardless of year, sex or size (model 1 in table 1; figure 2). Likewise, individuals that survived also had significantly lower feather T concentrations in all models (model 5 in table 1; figure 2). However, for T, the model with the highest support (model 5) indicates that this was owing to an interaction with body size (i.e. tarsus length; table 1). Feather T concentrations in individuals that died were more dependent on body size (steeper slope) than in animals that survived (table 1 and figure 4). Thus, birds that subsequently died had higher T concentrations that were positively associated with their body size, whereas in the birds that survived T concentrations were lower regardless of their body size.
= 3.6, p = 0.06). Individuals that survived also had significantly lower feather cortisol concentrations in all models tested, regardless of year, sex or size (model 1 in table 1; figure 2). Likewise, individuals that survived also had significantly lower feather T concentrations in all models (model 5 in table 1; figure 2). However, for T, the model with the highest support (model 5) indicates that this was owing to an interaction with body size (i.e. tarsus length; table 1). Feather T concentrations in individuals that died were more dependent on body size (steeper slope) than in animals that survived (table 1 and figure 4). Thus, birds that subsequently died had higher T concentrations that were positively associated with their body size, whereas in the birds that survived T concentrations were lower regardless of their body size.
Figure 2.
Feather concentrations (mean ± s.d.) of corticosterone, cortisol and testosterone in house sparrows that subsequently were confirmed to be alive (white bars) or inferred to have died (grey bars). All three steroids were significantly higher in the feathers of sparrows that subsequently died.
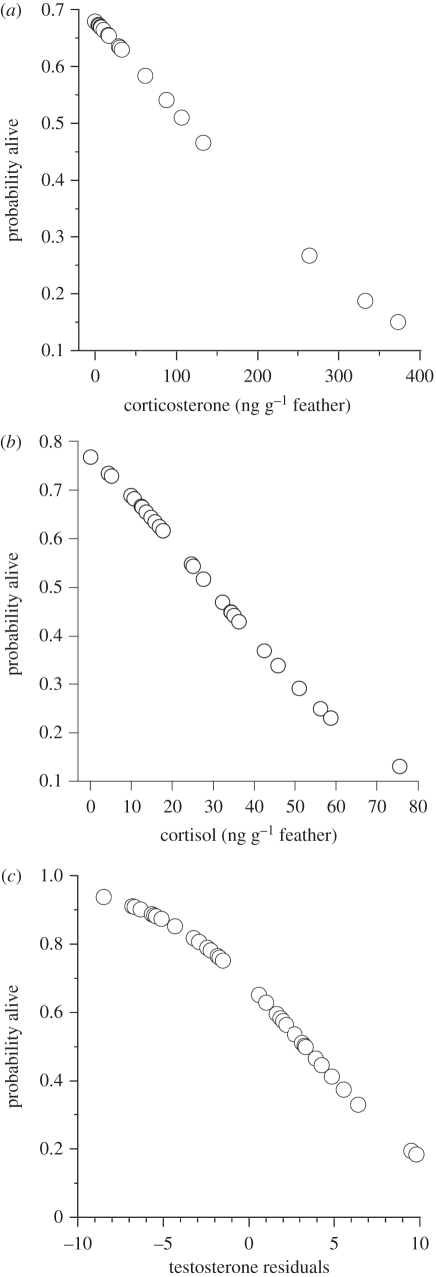
Figure 3.
Predicted values for the probability of survival, calculated by logistic regression, as a function of (a) feather corticosterone, (b) cortisol and (c) testosterone levels controlled for tarsus length.
Table 1.
Nominal logistic regression models for the effect of feather hormone, year, sex and tarsus length (proxy for size) on the survival of house sparrows. The whole-model statistic is provided in the first line of each model. The degree of freedom for each test and sample size for each feather hormone are in parentheses. The lowest AICc (italics) indicates the best model for each feather hormone. Significant effects are indicated in bold.
| effect | LR χ2 | p | AICc |
|---|---|---|---|
| corticosterone (49) | |||
| model 1 | 2.9 (1) | 0.087 | 65.8 |
| corticosterone | 2.9 (1) | 0.087 | |
| model 2 | 12.9 (2) | 0.002 | 58.1 |
| corticosterone | 7.6 (1) | 0.006 | |
| year | 9.9 (1) | 0.002 | |
| model 3 | 15.1 (4) | 0.005 | 60.7 |
| corticosterone | 6.9 (1) | 0.009 | |
| year | 10.3 (1) | 0.001 | |
| tarsus | 1.2 (1) | 0.256 | |
| sex | 1.4 (1) | 0.242 | |
| cortisol (49) | |||
| model 1 | 7.3 (1) | 0.007 | 61.4 |
| cortisol | 7.3 (1) | 0.007 | |
| model 2 | 11.0 (2) | 0.004 | 60.0 |
| cortisol | 5.7 (1) | 0.017 | |
| year | 3.6 (1) | 0.057 | |
| model 3 | 13.5 (4) | 0.009 | 62.3 |
| cortisol | 5.4 (1) | 0.020 | |
| year | 4.0 (1) | 0.046 | |
| tarsus | 0.9 (1) | 0.342 | |
| sex | 1.9 (1) | 0.168 | |
| testosterone (35) | |||
| model 1 | 5.7 (1) | 0.017 | 44.9 |
| testosterone | 5.7 (1) | 0.017 | |
| model 2 | 9.1 (2) | 0.011 | 43.9 |
| testosterone | 4.6 (1) | 0.032 | |
| year | 3.4 (1) | 0.065 | |
| model 3 | 22.7 (3) | < 0.0001 | 32.8 |
| testosterone | 17.3 (1) | < 0.0001 | |
| tarsus | 7.8 (1) | 0.005 | |
| tarsus × testosterone | 11.7 (1) | 0.001 | |
| model 4 | 26.5 (4) | < 0.0001 | 31.7 |
| testosterone | 19.1 (1) | < 0.0001 | |
| tarsus | 10.3 (1) | 0.001 | |
| tarsus × testosterone | 13.4 (1) | 0.0003 | |
| sex | 3.8 (1) | 0.051 | |
| model 5 | 30.0 (4) | < 0.0001 | 28.2 |
| testosterone | 19.8 (1) | < 0.0001 | |
| tarsus | 9.8 (1) | 0.002 | |
| tarsus × testosterone | 16.0 (1) | < 0.0001 | |
| year | 7.3 (1) | 0.007 |
Figure 4.
Feather testosterone levels were related to tarsus length (proxy for size) in house sparrows that were inferred to have died (filled symbols, solid trend line), but not in those that were confirmed to be alive (empty symbols, dashed trend line).
4. Discussion
In wild house sparrows, survival was inversely associated with GC and T concentrations in feathers. The feathers that we examined were formed during the autumn moult and therefore integrate steroid fluctuations over this interval, which follows the energetically demanding breeding period and precedes the energetically demanding winter months. Thus, feather steroid concentrations are biologically plausible parameters that are inversely linked to a direct measure of future individual fitness: subsequent survival. Moreover, we measured steroids using state-of-the-art LC-MS/MS, which permits the measurement of multiple steroids at one time and offers unmatched specificity.
(a). Glucocorticoids
Many studies, regardless of sex, species or breeding status, have attributed a negative association between survival and stress-induced circulating GC levels to the energetic costs associated with maintaining elevated GC levels [6–9,32,33]. In humans, cortisol levels in hair are a strong statistical predictor of future acute myocardial infarction [34]. The argument has also been made that GCs may facilitate behaviour that reduces mortality risk, thus improving survival [6]. However, in a majority of these studies, plasma GC levels were elevated by restraint, implants or hormonal stimulation. In the rare studies where baseline plasma GC levels were obtained, the association with survival has been inconsistent [6,35,36]. Here, we avoid confounding effects of the sampling procedure on the steroids of interest, and avoid relying on a single moment in time to represent dynamic steroid exposures, by using feather samples. This integrated measure supports the hypothesis that elevated GC concentrations reduce future survival [5].
Bortolotti et al. [15] have shown that feathers reflect circulating corticosterone levels and that surface washing does not remove corticosterone (electronic supplementary material of [15]). Since corticosterone is measured throughout the year in house sparrows [23,24,37,38], the source for corticosterone is assumed to be the adrenal gland. Using our analytical method, we also confirmed that plasma of male and female house sparrows contained corticosterone in both the breeding and non-breeding seasons (see electronic supplementary material). Thus, individuals with elevated adrenal secretion of corticosterone during the period of feather growth were less likely to survive subsequent winters. Our experimental design did not address the source of increased ‘stress’.
Samples tended to contain one, two or no detectable GCs at similar frequencies, with similar concentrations. Cortisol and corticosterone concentrations were associated; however, 70 to 80 per cent of the variance remains unexplained. As there is no ambiguity in cortisol quantitation by mass spectrometry, and no known mechanism for contamination of the feathers by cortisol, the origin of cortisol in plasma and feathers is assumed to be endogenous. There is evidence that avian adrenals can produce both corticosterone and cortisol [39,40], and a few studies report cortisol in avian plasma and tissues during development [41,42]. We detected a low level of cortisol in the plasma of breeding males, but none in plasma of moulting males or of females (see electronic supplementary material, figure S1). When cortisol was present in the plasma pool, corticosterone concentrations were approximately 50 times higher than cortisol concentrations. This contrasts with the feather results, in which concentrations of corticosterone and cortisol did not differ statistically.
Differences between plasma and feathers could be accounted for by differential binding of cortisol and corticosterone to binding globulins [23,43]. If cortisol had a lower binding globulin affinity than corticosterone, and was therefore over-represented in the free GC pool within plasma, then feathers would be cortisol-enhanced. It is also possible that feathers reflect concentrations of cortisol (and perhaps corticosterone) that are synthesized locally in the skin or feather follicle. Local GC synthesis occurs in mammalian skin and hair follicles, and human skin cells can synthesize both cortisol and corticosterone [44,45]. Local cortisol synthesis in skin could thereby be dissociated from adrenal corticosterone synthesis and might reflect an independent fitness-related trait, such as the ability to heal from injuries, cope with ectoparasites, or the extent of ultraviolet light exposure [46].
(b). Testosterone
The feathers we sampled reflect non-breeding T levels, which were, as expected, based on plasma T concentrations [20,21], similar in male and female house sparrows. In song sparrows (Melospiza melodia) and spotted antbirds (Hylophylax n. naevioides), aggression during the non-breeding period is maintained via androgens of adrenal, rather than gonadal, origin [47–49]. Similarly, in these house sparrow feathers, although gonadal synthesis or local T synthesis [50] remain possible, synthesis from circulating adrenal androgen precursors (such as dehydroepiandrosterone) is another likely source of T.
As with the GCs, high T concentrations have been hypothesized to be a risk to survival, but a benefit to reproductive success. Many of these hypotheses have been tested in the context of the ‘challenge hypothesis’ [11], with benefits of acute, rapid elevations in T but costs of chronic elevations in T. For example, in male dark-eyed juncos, survival is positively associated with maximal plasma T levels [51]. Even in humans, there is now evidence that men with higher T are more likely to marry, but that the birth of their child reduces that T [52]. Our results support the hypothesis that elevated T concentrations reduce future survival [13].
In our survival models, feather T levels significantly interacted with tarsus length. While a steeper slope was observed in the birds that died, we do not know the reason for this interaction. It is possible that normalizing selection might be acting by imposing fitness costs on having too much T for your size in spite of potential competitive advantages of higher T concentrations. A subsequent study has shown that supplemental feeding of house sparrow chicks results in longer tarsi in the Lundy population [53]. It is therefore possible that the larger sparrows that died had made an early commitment to growth, yet also tried to reproduce or grow beyond their energetic means, whereas the smaller birds that died might not have attempted reproduction. Interestingly, a recent study linked structural physiology and T with its finding that osteocalcin, which is a bone hormone, actively induces T production [54]. This discovery may indicate possible mechanisms for our observed interaction, but does not fully explain why we found that size affected T more notably in the birds that died.
(c). Correlations between glucocorticoids and testosterone
Correlations between the GCs and T in feathers were weak or absent. In contrast, a relationship between GCs and T has been documented in several systems. For example, in wild chimpanzees, plasma cortisol and T levels are synergistically and positively associated with parasite richness [55]. Evans et al. [56] found that, in house sparrows, T treatment increases plasma corticosterone levels and suppresses an immune response, but when the effects of corticosterone are controlled for, T is actually an immuno-enhancer, probably through its effect on dominance and access to resources. The dissociation between GCs and T in feathers may be owing to different sites of steroid synthesis during the moult (e.g. gonads, skin, brain or adrenals) or to different roles the steroids play in ecologically relevant contexts, which influence their incorporation into feathers.
5. Conclusions
Taken together, our results provide evidence that steroid levels in feathers, which integrate steroid concentrations over the period of feather growth, are biologically meaningful and are associated with survival outcomes. We also show that feather concentrations of cortisol are correlated with subsequent mortality, suggesting that cortisol is important in avian physiology. Further studies will be needed to determine the sites of steroid synthesis that result in feather incorporation, and whether relative risks for decreased survival are independent, or cumulative, across different steroids. Sampling of free-ranging birds through feathers, coupled to unambiguous steroid identification, is a promising approach for exploring associations between steroids and life-history traits, while avoiding the complicating effects of handling and capture on the steroids of interest.
Acknowledgements
We wish to thank Michal Sasson and Shay Barkan for helping with the plasma collection, and Lea Bond and Ella Ng for assistance with the LC-MS/MS analysis. We also thank the Lundy Company and their staff for allowing us to work on Lundy and for supporting our study. The field study and data analysis were supported by a New Zealand Top Achiever scholarship to S.N. and a grant from the UK Natural Environment Research Council to T.B. and S.N. Laboratory analyses were supported by an NSERC Discovery grant, and by infrastructure support from the Canadian Foundation for Innovation and the Alberta Education Fund (K.E.W.E.), as well as postdoctoral support for L.K. from the University of Calgary Faculty of Veterinary Medicine.
References
- 1.Salzet M., Vieau D., Day R. 2000. Crosstalk between nervous and immune systems through the animal kingdom: focus on opioids. Trends Neurosci. 23, 550–555 10.1016/S0166-2236(00)01642-8 (doi:10.1016/S0166-2236(00)01642-8) [DOI] [PubMed] [Google Scholar]
- 2.von Holst D. 1998. The concept of stress and its relevance for animal behavior. Stress Behav. 27, 1–131 10.1016/S0065-3454(08)60362-9 (doi:10.1016/S0065-3454(08)60362-9) [DOI] [Google Scholar]
- 3.Cooper E. L., Faisal M. 1990. Phylogenetic approach to endocrine–immune system interactions. J. Exp. Zool. 256, 46–52 10.1002/jez.1402560409 (doi:10.1002/jez.1402560409) [DOI] [PubMed] [Google Scholar]
- 4.Jasnow A. M., Drazen D. L., Huhman K. L., Nelson R. J., Demas G. E. 2001. Acute and chronic social defeat suppresses humoral immunity of male Syrian hamsters (Mesocricetus auratus). Horm. Behav. 40, 428–433 10.1006/hbeh.2001.1708 (doi:10.1006/hbeh.2001.1708) [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky R. M. 2000. Stress hormones: good and bad. Neurobiol. Dis. 7, 540–542 10.1006/nbdi.2000.0350 (doi:10.1006/nbdi.2000.0350) [DOI] [PubMed] [Google Scholar]
- 6.Hau M., Ricklefs R. E., Wikelski M., Lee K. A., Brawn J. D. 2010. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212 10.1098/rspb.2010.0673 (doi:10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blas J., Bortolotti G. R., Tella J. L., Baos R., Marchant T. A. 2007. Stress response during development predicts fitness in a wild, long lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884 10.1073/pnas.0700232104 (doi:10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDougall-Shackleton S. A., Dindia L., Newman A. E. M., Potvin D. A., Stewart K. A., MacDougall-Shackleton E. A. 2009. Stress, song and survival in sparrows. Biol. Lett. 5, 746–748 10.1098/rsbl.2009.0382 (doi:10.1098/rsbl.2009.0382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero L. M., Wikelski M. 2001. Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Niño events. Proc. Natl Acad. Sci. USA 98, 7366–7370 10.1073/pnas.131091498 (doi:10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staub N. L., DeBeer M. 1997. The role of androgens in female vertebrates. Gen. Comp. Endocrinol. 108, 1–24 10.1006/gcen.1997.6962 (doi:10.1006/gcen.1997.6962) [DOI] [PubMed] [Google Scholar]
- 11.Wingfield J. C., Hegner R. E., Dufty A. M., Ball G. F. 1990. The challenge hypothesis: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 10.1086/285134 (doi:10.1086/285134) [DOI] [Google Scholar]
- 12.Wingfield J. C., Lynn S. E., Soma K. K. 2001. Avoiding the ‘costs' of testosterone: ecological bases of hormone–behavior interactions. Brain Behav. Evol. 57, 239–251 10.1159/000047243 (doi:10.1159/000047243) [DOI] [PubMed] [Google Scholar]
- 13.Ketterson E. D., Nolan V. 1992. Hormones and life histories: an integrative approach. Am. Nat. 140, S33–S62 10.1086/285396 (doi:10.1086/285396) [DOI] [PubMed] [Google Scholar]
- 14.Hamilton J. B. 1948. The role of testicular secretions as indicated by the effects of castration in man and by studies of pathological condition and the short lifespan associated with maleness. Recent Prog. Horm. Res. 3, 257–322 [Google Scholar]
- 15.Bortolotti G. R., Marchant T. A., Blas J., German T. 2008. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 22, 494–500 10.1111/j.1365-2435.2008.01387.x (doi:10.1111/j.1365-2435.2008.01387.x) [DOI] [Google Scholar]
- 16.de Kloet E. R., Joels M., Holsboer F. 2005. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475 10.1038/nrn1683 (doi:10.1038/nrn1683) [DOI] [PubMed] [Google Scholar]
- 17.Cleasby I. R., Nakagawa S., Gillespie D. O. S., Burke T. 2010. The influence of sex and body size on nestling survival and recruitment in the house sparrow. Biol. J. Linn. Soc. 101, 680–688 10.1111/j.1095-8312.2010.01515.x (doi:10.1111/j.1095-8312.2010.01515.x) [DOI] [Google Scholar]
- 18.Nakagawa S., Ockendon N., Gillespie D. O. S., Hatchwell B. J., Burke T. 2007. Does the badge of status influence parental care and investment in house sparrows? An experimental test. Oecologia 153, 749–760 10.1007/s00442-007-0765-4 (doi:10.1007/s00442-007-0765-4) [DOI] [PubMed] [Google Scholar]
- 19.Anderson T. R. 2006. Biology of the ubiquitous house sparrow: from genes to populations, pp. 547 New York, NY: Oxford University Press [Google Scholar]
- 20.Hegner R. E., Wingfield J. C. 1986. Gonadal development during autumn and winter in house sparrows. Condor 88, 269–278 10.2307/1368872 (doi:10.2307/1368872) [DOI] [Google Scholar]
- 21.Hegner R. E., Wingfield J. C. 1987. Social status and circulating levels of hormones in flocks of house sparrows Passer domesticus. Ethology 76, 1–14 10.1111/j.1439-0310.1987.tb00667.x (doi:10.1111/j.1439-0310.1987.tb00667.x) [DOI] [Google Scholar]
- 22.Romero L. M. 2006. Seasonal changes in hypothalamic-pituitary-adrenal axis sensitivity in free-living house sparrows (Passer domesticus). Gen. Comp. Endocrinol. 149, 66–71 10.1016/j.ygcen.2006.05.011 (doi:10.1016/j.ygcen.2006.05.011) [DOI] [PubMed] [Google Scholar]
- 23.Romero L. M., Cyr N. E., Romero R. C. 2006. Corticosterone responses change seasonally in free-living house sparrows (Passer domesticus). Gen. Comp. Endocrinol. 149, 58–65 10.1016/j.ygcen.2006.05.004 (doi:10.1016/j.ygcen.2006.05.004) [DOI] [PubMed] [Google Scholar]
- 24.Ouyang J. Q., Sharp P. J., Dawson A., Quetting M., Hau M. 2011. Hormone levels predict individual differences in reproductive success in a passerine bird. Proc. R. Soc. B 278, 2537–2545 10.1098/rspb.2010.2490 (doi:10.1098/rspb.2010.2490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson L. 1992. Identification guide to European passerines. Thetford, UK: BTO [Google Scholar]
- 26.McDonald P. G., Griffith S. C. 2011. To pluck or not to pluck: the hidden ethical and scientific costs of relying on feathers as a primary source of DNA. J. Avian Biol. 42, 197–203 10.1111/j.1600-048X.2011.05365.x (doi:10.1111/j.1600-048X.2011.05365.x) [DOI] [Google Scholar]
- 27.Schroeder J., Cleasby I. R., Nakagawa S., Ockendon N., Burke T. 2011. No evidence for adverse effects on fitness of fitting passive integrated transponders (PITs) in wild house sparrows Passer domesticus. J. Avian Biol. 42, 1–5 10.1111/j.1600-048x.2010.05271.x (doi:10.1111/j.1600-048x.2010.05271.x) [DOI] [Google Scholar]
- 28.Stanczyk F. Z., Clarke N. J. 2010. Advantages and challenges of mass spectrometry assays for steroid hormones. J. Steroid Biochem. Mol. Biol. 121, 491–495 10.1016/j.jsbmb.2010.05.001 (doi:10.1016/j.jsbmb.2010.05.001) [DOI] [PubMed] [Google Scholar]
- 29.Soldin S. J., Soldin O. P. 2009. Steroid hormone analysis by tandem mass spectrometry. Clin. Chem. 55, 1061–1066 10.1373/clinchem.2007.100008 (doi:10.1373/clinchem.2007.100008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnham K. P., Anderson D. R. 2001. Kullback–Leibler information as a basis for strong inference in ecological studies. Wildlife Res. 28, 111–119 10.1071/WR99107 (doi:10.1071/WR99107) [DOI] [Google Scholar]
- 31.Johnson J. B., Omland K. S. 2004. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108 10.1016/j.tree.2003.10.013 (doi:10.1016/j.tree.2003.10.013) [DOI] [PubMed] [Google Scholar]
- 32.Goutte A., Angelier F., Welcker J., Moe B., Clement-Chastel C., Gabrielsen G. W., Bech C., Chastel O. 2010. Long-term survival effect of corticosterone manipulation in black-legged kittiwakes. Gen. Comp. Endocrinol. 167, 246–251 10.1016/j.ygcen.2010.03.018 (doi:10.1016/j.ygcen.2010.03.018) [DOI] [PubMed] [Google Scholar]
- 33.Cote J., Clobert J., Meylan S., Fitze P. S. 2006. Experimental enhancement of corticosterone levels positively affects subsequent male survival. Horm. Behav. 49, 320–327 10.1016/j.yhbeh.2005.08.004 (doi:10.1016/j.yhbeh.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 34.Pereg D., Gow R., Mosseri M., Lishner M., Rieder M., Van Uum S., Koren G. 2011. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress: Int. J. Biol. Stress 14, 73–81 10.3109/10253890.2010.511352 (doi:10.3109/10253890.2010.511352) [DOI] [PubMed] [Google Scholar]
- 35.Ninnes C. E., et al. 2011. Environmental influences on Adelie penguin breeding schedules, endocrinology, and chick survival. Gen. Comp. Endocrinol. 173, 139–147 10.1016/j.ygcen.2011.05.006 (doi:10.1016/j.ygcen.2011.05.006) [DOI] [PubMed] [Google Scholar]
- 36.Comendant T., Sinervo B., Svensson E. I., Wingfield J. 2003. Social competition, corticosterone and survival in female lizard morphs. J. Evol. Biol. 16, 948–955 10.1046/j.1420-9101.2003.00598.x (doi:10.1046/j.1420-9101.2003.00598.x) [DOI] [PubMed] [Google Scholar]
- 37.Li D. M., Wang G., Wingfield J. C., Zhang Z., Ding C. Q., Lei F. M. 2008. Seasonal changes in adrenocortical responses to acute stress in Eurasian tree sparrow (Passer montanus) on the Tibetan Plateau: comparison with house sparrow (P. domesticus) in North America and with the migratory P. domesticus in Qinghai Province. Gen. Comp. Endocrinol. 158, 47–53 10.1016/j.ygcen.2008.06.002 (doi:10.1016/j.ygcen.2008.06.002) [DOI] [PubMed] [Google Scholar]
- 38.Romero L. M., Rich E. L. 2007. Photoperiodically-induced changes in hypothalamic–pituitary–adrenal axis sensitivity in captive house sparrows (Passer domesticus). Comp. Biochem. Physiol. A 147, 562–568 10.1016/j.cbpa.2007.02.004 (doi:10.1016/j.cbpa.2007.02.004) [DOI] [PubMed] [Google Scholar]
- 39.Schmidt K. L., Malisch J. L., Breuner C. W., Soma K. K. 2010. Corticosterone and cortisol binding sites in plasma, immune organs and brain of developing zebra finches: intracellular and membrane-associated receptors. Brain Behav. Immun. 24, 908–918 10.1016/j.bbi.2010.02.008 (doi:10.1016/j.bbi.2010.02.008) [DOI] [PubMed] [Google Scholar]
- 40.Schmidt K. L., Pradhan D. S., Shah A. H., Charlier T. D., Chin E. H., Soma K. K. 2008. Neurosteroids, immunosteroids, and the balkanization of endocrinology. Gen. Comp. Endocrinol. 157, 266–274 10.1016/j.ygcen.2008.03.025 (doi:10.1016/j.ygcen.2008.03.025) [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T., Tanabe Y., Hirano H. 1978. Evidence of in vitro formation of cortisol by adrenal gland of embryonic and young chickens (Gallus domesticus). Gen. Comp. Endocrinol. 35, 302–308 10.1016/0016-6480(78)90076-x (doi:10.1016/0016-6480(78)90076-x) [DOI] [PubMed] [Google Scholar]
- 42.Jenkins S. A., Porter T. E. 2004. Ontogeny of the hypothalamo–pituitary–adrenocortical axis in the chicken, embryo: a review. Domestic Anim. Endocrinol. 26, 267–275 10.1016/j.domaniend.2004.01.001 (doi:10.1016/j.domaniend.2004.01.001) [DOI] [PubMed] [Google Scholar]
- 43.Breuner C. W., Orchinik M. 2002. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 175, 99–112 10.1677/joe.0.1750099 (doi:10.1677/joe.0.1750099) [DOI] [PubMed] [Google Scholar]
- 44.Taves M. D., Gomez-Sanchez C. E., Soma K. K. 2011. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am. J. Physiol. Endocrinol. Metab. 301, E11–E24 10.1152/ajpendo.00100.2011 (doi:10.1152/ajpendo.00100.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito N., Ito T., Kromminga A., Bettermann A., Takigawa M., Kees F., Straub R. H., Paus R. 2005. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal (HPA) axis and synthesize cortisol. FASEB J. 19, 1332–1358 10.1096/fj.04-1968fje (doi:10.1096/fj.04-1968fje) [DOI] [PubMed] [Google Scholar]
- 46.Skobowiat C., Dowdy J. C., Sayre R. M., Tuckey R. C., Slominski A. 2011. Cutaneous hypothalamic–pituitary–adrenal axis homolog: regulation by ultraviolet radiation. Am. J. Physiol. Endocrinol. Metab. 301, E484–E493 10.1152/ajpendo.00217.2011 (doi:10.1152/ajpendo.00217.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soma K. K., Wingfield J. C. 2001. Dehydroepiandrosterone in songbird plasma: seasonal regulation and relationship to territorial aggression. Gen. Comp. Endocrinol. 123, 144–155 10.1006/gcen.2001.7657 (doi:10.1006/gcen.2001.7657) [DOI] [PubMed] [Google Scholar]
- 48.Newman A. E. M., Pradhan D. S., Soma K. K. 2008. Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinology 149, 2537–2545 10.1210/en.2007-1363 (doi:10.1210/en.2007-1363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hau M., Beebe K. 2011. Plastic endocrine regulation of year-round territorial aggression in tropical male spotted antbirds. Gen. Comp. Endocrinol. 172, 305–313 10.1016/j.ygcen.2011.03.016 (doi:10.1016/j.ygcen.2011.03.016) [DOI] [PubMed] [Google Scholar]
- 50.Schlinger B. A., Fivizzani A. J., Callard G. V. 1989. Aromatase, 5-alpha-reductase and 5-β-reductase in brain, pituitary and skin of the sex-role reversed Wilsons phalarope. J. Endocrinol. 122, 573–581 10.1677/joe.0.1220573 (doi:10.1677/joe.0.1220573) [DOI] [PubMed] [Google Scholar]
- 51.McGlothlin J. W., Whittaker D. J., Schrock S. E., Gerlach N. M., Jawor J. M., Snajdr E. A., Ketterson E. D. 2010. Natural selection on testosterone production in a wild songbird population. Am. Nat. 175, 687–701 10.1086/652469 (doi:10.1086/652469) [DOI] [PubMed] [Google Scholar]
- 52.Gettler L. T., McDadea T. W., Feranilc A. B., Kuzawa C. W. 2011. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc. Natl Acad. Sci. USA 108, 16 194–16 199 10.1073/pnas.1105403108 (doi:10.1073/pnas.1105403108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cleasby I. R., Burke T., Schroeder J., Nakagawa S. 2011. Food supplements increase adult tarsus length, but not growth rate, in an island population of house sparrows (Passer domesticus). BMC Res. Notes 4, 43122018144 [Google Scholar]
- 54.Oury F., et al. 2011. Endocrine regulation of male fertility by the skeleton. Cell 144, 796–809 10.1016/j.cell.2011.02.004 (doi:10.1016/j.cell.2011.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muehlenbein M. P. 2006. Intestinal parasite infections and fecal steroid levels in wild chimpanzees. Am. J. Phys. Anthropol. 130, 546–550 10.1002/ajpa.20391 (doi:10.1002/ajpa.20391) [DOI] [PubMed] [Google Scholar]
- 56.Evans M. R., Goldsmith A. R., Norris S. R. A. 2000. The effects of testosterone on antibody production and plumage coloration in male house sparrows (Passer domesticus). Behav. Ecol. Sociobiol. 47, 156–163 10.1007/s002650050006 (doi:10.1007/s002650050006) [DOI] [Google Scholar]