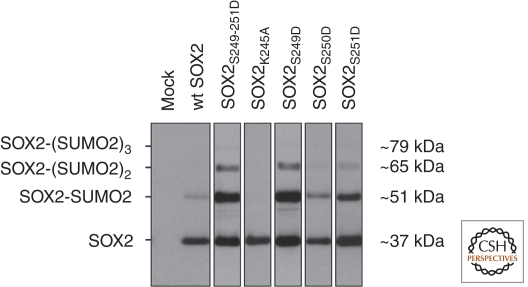
Figure 2.
Western blots showing wild-type SOX2 and SOX2 mutants expressed in HeLa cells. HeLa cells that were transfected with 6 × histidine-tagged SUMO2 in combination with either wild-type SOX2 (lane 2) or SOX2 mutants where serine residues 249, 250, and 251 were all (lane 3) or individually (lanes 5–7) mutated to aspartic acid residues, which mimics constitutive phosphorylation of the respective serine residues. SUMO2-SOX2 complexes were purified from the cell lysates with Ni-NTA beads binding the 6 × histidine tag of SUMO2, and then subjected to SDS-PAGE and Western blotting using a SOX2-specific antibody. Wild-type SOX2 (lane 2) is marginally SUMOylated; mutating lysine 245 into alanine (lane 4) shows that this residue is the SUMO target site. Whereas mutation of serine 250 into aspartic acid (lane 6) has no pronounced effect, mutating either all three serine residues simultaneously (lane 3) or serine 249 (lane 5) or 251 (lane 7) individually results in increased polySUMOylation. Mock: negative control of HeLa cells transfected with an empty plasmid (D Van Hoof, J Krijgsveld, and CL Mummery, unpubl.).