Abstract
We report a rare synchronous presentation of adrenocortical carcinoma (ACC) and papillary thyroid carcinoma (PTC). A 31-year-old male first presented with a large left adrenal mass that was identified during the workup for refractory hypertension due to hyperaldosteronism. The mass was removed surgically with pathology showing ACC. The patient was then treated with adjuvant radiation therapy and mitotane chemotherapy. Four months post ACC resection, metastatic ACC to the right upper lung and PTC in the left lobe of the thyroid were found in surveillance imaging. He subsequently developed pulmonary, contralateral adrenal and brain metastases from his ACC. Li Fraumeni syndrome and Multiple Endocrine Neoplasia Type I (MEN I) were considered, but testing of both P53 and menin genes showed no mutation. We also performed a review of the literature and found three similar cases, however gene mutation analysis was not performed..
Key words: adrenocortical carcinoma, papillary thyroid carcinoma, hereditary cancer syndrome.
Introduction
In this report we review the case of a 31-year-old male diagnosed with an adrenocortical carcinoma (ACC) and a papillary thyroid carcinoma (PTC). Adrenocortical carcinomas are an extremely rare type of cancer, with an incidence of less than 0.2 per 100,000 in the U.S.1 Thyroid cancer is more common with an incidence rate of 11.0 per 100,000 per year in the U.S.; papillary carcinoma accounts for approximately 85% of all thyroid cancers and is 3 times more common in women than men.2 Given the presentation of these rare tumors together in a young patient, their appearance is suggestive of a possible hereditary link. Of the known syndromes caused by a hereditary predisposition to multiple endocrine tumors, none specifically include the occurrence of PTC with ACC.3 Of the hereditary conditions that present with the tumors in our case, Multiple Endocrine Neoplasia (MEN) Type 1 is known to present with more than 20 possible combinations of endocrine and non-endocrine tumors, including ACC but not PTC.4 Additionally, MEN Type 2 syndromes involve pheochromocytomas and medullary thyroid cancer, but do not traditionally involve PTC or ACC in the established syndrome.3 Adrenocortical carcinomas are also known to appear in Li-Fraumeni syndrome (LFS), which is associated with an autosomal dominant mutation in the TP53 gene that is the source of tumors at an early age.5 There are few case reports in the literature that present with ACC and PTC. The available reports attribute the concomitant appearance of the tumors to coincidence, but do not discount a potential genetic or hereditary link.6–8 Thus, this case represents an atypical combination of endocrine tumors with a potential hereditary component.
Case Report
A 31-year-old, previously healthy, white male presented to the emergency department with a nine-month history of intermittent fevers, headaches and muscle aches occurring in isolated episodes approximately a month apart, a 5-day history of back pain, and acute onset fevers to 38.9° Celsius. Past medical history included gastroesophageal reflux disease (GERD), controlled with lansoprazole. He was taking no other medications prior to his initial presentation. He was working as a construction supervisor and had smoked half of a pack of cigarettes per day for the past 12 years. Significant family history included a father with hypertension and congestive heart failure, paternal grandmother with leukemia, paternal aunt with non-Hodgkin's lymphoma, maternal uncle with multiple myeloma, and a maternal great grandmother with metastatic colon cancer. A physical exam revealed severe hypertension (210/110), no palpable abdominal masses and was otherwise unremarkable. He was prescribed atenolol (50 mg daily), which initially controlled the high blood pressure.
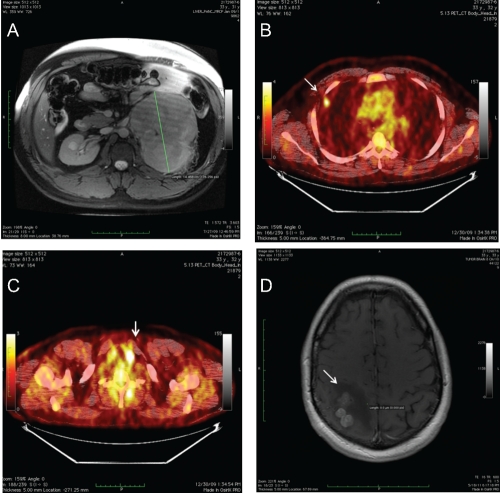
However, the hypertension proved refractory to treatment for four months, even while taking three anti-hypertensive drugs simultaneously (atenolol, valsartan and amlodipine). A more thorough work-up was initiated. An abdominal ultrasound revealed a large left adrenal mass (Figure 1A). A follow-up CT scan confirmed a 14.5×14.4×9.8cm left adrenal mass displacing the left superior pole of the kidney. It was suspected that the severe hypertension was secondary to a hormone-secreting tumor, therefore cortisol and aldosterone levels were checked prior to surgical resection of the mass. Urine metanephrine, normetanephrine and serum levels were normal; aldosterone levels were slightly elevated, while the aldosterone/renin activity ratio was above normal limits at 112.5 (ref. ≤25.0), indicating hyperaldosteronism.
Figure 1.
A) Left adrenal mass, MRI; B) Right upper lobe pulmonary nodule, PETCT Scan; C) Thyroid nodule, PETCT Scan; D) R parietal brain metastases and associated edema, MRI with contrast.
Surgical resection of the adrenal mass was performed; pathology revealed a 19.5 cm adrenocortical carcinoma with large vessel invasion. The tumor was less than 1 mm from the resection margin, but was surrounded by a thin fibrous capsule and demonstrated no definitive evidence of capsular extension or invasion. The tumor was staged as pT2 with no lymph nodes analyzed. The patient recovered from surgery with no major complications. Post-operative CT scan revealed a left pleural effusion, but was negative for metastatic disease. Mild hypertension persisted, managed with 100 mg atenolol. Within two months following surgery, aldosterone levels returned to normal.
Six weeks following the operation, the patient began adjuvant radiation concurrent with mitotane. He received a total dose of 54 Gy at 2 Gy/fraction given over 5.5 weeks to the resection bed and preoperative tumor volume. Following completion of radiation, he continued on single agent mitotane chemotherapy.
Four months following the original adrenalectomy, a surveillance CT scan revealed a 1 cm nodule in the right upper lung. A subsequent PETCT showed an FDG avid nodule (SUV 3.7) in the right upper lung and a 4 mm FDG avid left thyroid nodule (SUV 13.7) (Figure 1B, 1C). The FDG avid lung nodule was thought to represent metastatic disease (ACC vs thyroid). The patient underwent thoracoscopic right upper and middle pulmonary lobe wedge resections; pathology showed a 1.5 cm tumor consistent with metastatic ACC with a negative resection margin. Additionally, an ultrasound guided fine needle aspiration (FNA) of the thyroid nodule was performed; pathology showed PTC. Six months following the original adrenalectomy and 2 months following the thoracoscopic pulmonary wedge resection and thyroid FNA, a short interval surveillance PETCT scan showed no evidence of metastatic disease but re-confirmed an FDG avid left thyroid nodule. Given absence of further metastatic disease, the patient underwent a total thyroidectomy; pathology revealed multifocal PTC with positive margins. The patient was started on synthroid and I-131 radioactive iodine remnant ablation was performed. The patient continued on single agent mitotane for a total of 8 months (6 weeks concurrent with radiation and 6 months as a single agent). The maximum tolerated dose was 1500 mg daily; therapeutic drug levels of 10–14 mg/L were not reached due to GI side effects. The medication was stopped given rising creatinine and concerns for renal toxicity.
Eleven months following his original adrenalectomy and 7 months following the thoracoscopic pulmonary wedge resection, the patient underwent a surveillance PETCT scan which demonstrated a new 1 cm nodule in the right upper lobe (SUV 5.2). The patient then underwent a redo thoracoscopic right upper lobe wedge resection; pathology was consistent with fibrotic scar with no evidence of carcinoma.
Eighteen months following his original adrenalectomy and 14 months following the original pulmonary lobe wedge resection, another surveillance PETCT scan demonstrated a new 4.5×5.3×4.7 cm right upper lobe mass (SUV 8.3) a hypermetabolic 1.2 cm pleural based mass, and a hypermetabolic nodular right adrenal gland. He underwent a right thoracoscopic converted to open thoracotomy for right upper lobectomy; pathology revealed metastatic ACC 5 cm with negative margins, in addition to a pleural-based metastasis. A postoperative PETCT unfortunately showed rapid disease progression in the right hilum and right adrenal gland. The patient was started on systemic chemotherapy with 5-fluorouracil (5FU) and adriamycin. After one cycle of 5FU and adriamycin, the patient developed non-focal neurologic changes. A brain MRI showed multiple right parietal small lobulated parenchymal brain enhancing lesions with surrounding vasogenic edema (Figure 1D); there were additionally several small left temporal occipital metastases. A craniotomy and resection of parietal lesions were performed; pathology revealed metastatic adrenocortical carcinoma. He subsequently underwent stereotactic radiosurgery to the resection bed. The patient was then started on temozolomide and capecitabine chemotherapy. In a matter of weeks, the patient developed recurrent brain metastases and additional metastases to bone and multimple soft tissue sites. Despite whole brain radiotherapy the patient had progessive disease, a worsening performance status and died, two years from his original diagnosis.
Genetic testing was pursued to rule out known hereditary conditions that could potentially cause the combination of ACC and PTC, specifically LFS and MEN I. Gene mutation analysis revealed a wild-type p53 gene. Analysis of the menin gene also revealed no mutation. No further genetic testing was obtained. Germline DNA was stored for future research based whole genome sequencing.
Literature review
We performed a Medline search for cases presenting with adrenocortical carcinomas or adenomas and papillary thyroid carcinomas. We found three cases that matched closely with our patient (Table 1).
Table 1. Literature review of case reports containing papillary thyroid carcinoma and adrenal lesions.
Fukushima et al.6 reported a 45-year-old female with a virilizing adrenocortical adenoma, a papillary thyroid carcinoma, and a benign pancreatic nodule. The authors considered the concomitant occurrence of multiple endocrine lesions to be coincidental given the lack of family history. However, the possibility for MEN was considered, yet no genetic testing was performed. Casula et al.7 reported the case of a 39-year-old male who was found to have a locally advanced papillary thyroid carcinoma and an aldosterone-secreting adrenocortical adenoma. Again, the association of the tumors was ruled to be most likely coincidental, however MEN was considered despite no family history or genetic testing. Noordzig, et al.8 described four cases where a thyroid neoplasm was discovered by PETCT in the course of care for another primary lesion. One case was that of a 64-year-old woman initially found to have an adrenocortical carcinoma, who also had a papillary thyroid carcinoma discovered by PETCT imaging and biopsy. Again, genetic testing was not performed.
Discussion
In summary, we reported the case of a 31-year-old male who initially presented with severe hypertension and was found to have an aldosterone-secreting metastatic adrenocortical carcinoma. After resection of the ACC, the patient was found to have a localized PTC and subsequently developed metastatic ACC to the lungs and brain. Of primary concern for this case was the appearance of two endocrine tumors within a short period of time and the possibility of an underlying hereditary cancer syndrome. Both tumors are relatively rare in the general population, and their appearance together seemed suggestive of a hereditary component, however tests for LFS and MEN I both returned negative.
Adrenocortical carcinomas are rare and typically have a poor prognosis. There are varying reports on the percentage of ACCs that are functioning, with some papers indicating as many as 79% of ACCs as functioning – producing one or more adrenal hormones.9 Most functioning ACCs produce glucocorticoids. ACC often presents with hypertension; primary hyperaldosteronism is less common. The most effective treatment for ACC is complete resection ,10 which was performed in the case of our patient. In cases of incomplete resection or non-operated tumors, survival is often less than one year.11–13 Survival varies based on extent of disease, with Stage IV disease showing a five-year survival of 22%.12
Studies of adjuvant treatment following resection of ACCs demonstrate mixed results. A retrospective study by Terzolo et al. included 56 centers and 177 patients with ACC in Germany and Italy showed recurrence free-survival was extended to 42 months in the group receiving adjuvant mitotane chemotherapy as opposed to 10 months in the control groups.14 Other retrospective studies of adjuvant mitotane therapy have shown no advantage over surgical resection alone.15,16 However, these studies were primarily conducted at single centers and did not contain as many patients compared to the Terzolo study. There is an ongoing prospective phase III study (ADI-UVO study) of patients with ACC comparing adjuvant mitotane vs. follow-up that will hopefully offer greater clarity on the benefits of adjuvant mitotane therapy (NCT00777244).
The role of adjuvant radiation is also ill-defined, but studies have suggested that the risk of local recurrence, particularly with larger tumors, can be quite high. In a matched-pair analysis by Fassnacht et al., the investigators reported a 79% local control in 14 patients who received adjuvant radiotherapy compared with 12% (P<0.01) in 14 patients matched for stage, resection status, the use of adjuvant mitotane, and tumor size.17 Sabolch et al. demonstrated 4.7 times risk of local failure (P=0.03) in 58 patients treated for primary disease and recurrent disease who did not receive adjuvant radiotherapy.18 Recommendations have been proposed by the investigators from University Hospital Wuerzberg are for adjuvant radiation for tumors ≥8 cm or for tumors with questionable margin status.19 A minimum dose of 40 Gy should be given and doses between 50-60 Gy should be considered using standard fractionation. Radiotherapy was given in this patient due to the large size of the tumor and the very close margin.
Papillary thyroid carcinomas are much less aggressive than ACC and therefore have a better prognosis post-resection. The primary treatment for PTC is surgical resection. Since our patient had multifocal disease, a total thyroidectomy was performed to reduce risk of recurrence. About 15% of patients with PTC will experience relapse following surgical resection of the thyroid and approximately 5% of all PTC cases are lethal.20 Cause specific survival for patients undergoing total thyroidectomy is reported at 100% in some studies for patients with less advanced disease.21 However, the combined presentation of PTC and ACC complicates the prognosis for our patient.
Most ACCs and PTCs are sporadic, however, given the occurrence of two different endocrine neoplasms within a short period of time and the young age of our patient, the possibility of a hereditary cancer syndrome was considered. The most likely hereditary syndromes for this case associated with ACC were MEN (mutation in menin), and LFS (mutation in the TP53). While no hereditary cancer syndromes were identified in this case, this does not preclude the possibility of an untested or unknown disorder.
References
- 1.Fassnacht M, Kreissl MC, Weismann D, Allolio B. New targets and therapeutic approaches for endocrine malignancies. Pharmacol Ther. 2009;123:117–41. doi: 10.1016/j.pharmthera.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, et al. National Cancer Institute; Bethesda, MD: 2011. SEER Cancer Statistics Review, 1975-2008.http://seer.cancer.gov/csr/1975_2008/ based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Offit K. Abeloff's Clinical Oncology. 4th Ed: Churchill Livingston; 2008. Genetic Factors: Hereditary Cancer Predispostion Syndromes. [Google Scholar]
- 4.Falchetti A, Marini F, Luzi E, et al. Multiple endocrine neoplasia type 1 (MEN1): not only inherited endocrine tumors. Genet Med. 2009;11:825–35. doi: 10.1097/GIM.0b013e3181be5c97. [DOI] [PubMed] [Google Scholar]
- 5.Ford JM, Kastan M. Abeloff's Clinical Oncology. 4th Ed. Philadelphia PA:: Churchill Livingston; 2008. DNA Damage Response Pathways and Cancer. [Google Scholar]
- 6.Fukushima A, Okada Y, Tanikawa T, et al. Virilizing adrenocortical adenoma with Cushing's syndrome, thyroid papillary carcinoma and hypergastrinemia in a middle-aged woman. Endocr J. 2003;50:179–87. doi: 10.1507/endocrj.50.179. [DOI] [PubMed] [Google Scholar]
- 7.Casula G, Angioy F, Sirigu P, Sirigu F. Carcinoma of the thyroid gland, adenoma of the adrenal cortex and peptic ulcer: an unreported association. Tumori. 1976;62:665–72. doi: 10.1177/030089167606200610. [DOI] [PubMed] [Google Scholar]
- 8.Noordzij MJ, de Heide LJ, Links TP, et al. [Four patients with incidentalomas of the thyroid discovered on 18-fluoro-deoxyglucose positron-emission tomography (FDG-PET)] Ned Tijdschr Geneesk. 2007;151:2337–41. [PubMed] [Google Scholar]
- 9.Roman S. Adrenocortical carcinoma. Curr Opin Oncol. 2006;18:36–42. doi: 10.1097/01.cco.0000198976.43992.14. [DOI] [PubMed] [Google Scholar]
- 10.Lacroix A. Approach to the patient with adrenocortical carcinoma. J Clin Endocrinol Metab. 2010;95:4812–22. doi: 10.1210/jc.2010-0990. [DOI] [PubMed] [Google Scholar]
- 11.Borrelli D, Bergamini C, Borrelli A, et al. [Surgical strategy in the treatment of adrenal cortex cancer. Expanded and repeated interventions] Ann Ital Chir. 2003;74:311–7. [PubMed] [Google Scholar]
- 12.Icard P, Chapuis Y, Andreassian B, et al. Adrenocortical carcinoma in surgically treated patients: a retrospective study on 156 cases by the French Association of Endocrine Surgery. Surgery. 1992;112:972–9 discussion. 9–80. [PubMed] [Google Scholar]
- 13.Lee JE, Berger DH, el-Naggar AK, et al. Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery. 1995;118:1090–8. doi: 10.1016/s0039-6060(05)80119-9. [DOI] [PubMed] [Google Scholar]
- 14.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–80. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 15.Barzon L, Fallo F, Sonino N, et al. Adrenocortical carcinoma: experience in 45 patients. Oncology. 1997;54:490–6. doi: 10.1159/000227608. [DOI] [PubMed] [Google Scholar]
- 16.Vassilopoulou-Sellin R, Guinee VF, Klein MJ, et al. Impact of adjuvant mitotane on the clinical course of patients with adrenocortical cancer. Cancer. 1993;71:3119–23. doi: 10.1002/1097-0142(19930515)71:10<3119::aid-cncr2820711037>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Fassnacht M, Hahner S, Polat B, et al. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adreno-cortical carcinoma. J Clin Endocrinol Metab. 2006;91:4501–4. doi: 10.1210/jc.2006-1007. [DOI] [PubMed] [Google Scholar]
- 18.Sabolch A, Feng M, Griffith K, et al. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2011;80:1477–84. doi: 10.1016/j.ijrobp.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Polat B, Fassnacht M, Pfreundner L, et al. Radiotherapy in adrenocortical carcinoma. Cancer. 2009;115:2816–23. doi: 10.1002/cncr.24331. [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg H. Williams Textbook of Endocrinology. 11th Ed. Philadelpia, PA: Saunders; 2008. Papillary Thyroid Cancer. [Google Scholar]
- 21.Palme CE, Waseem Z, Raza SN, et al. Management and outcome of recurrent well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:819–24. doi: 10.1001/archotol.130.7.819. [DOI] [PubMed] [Google Scholar]