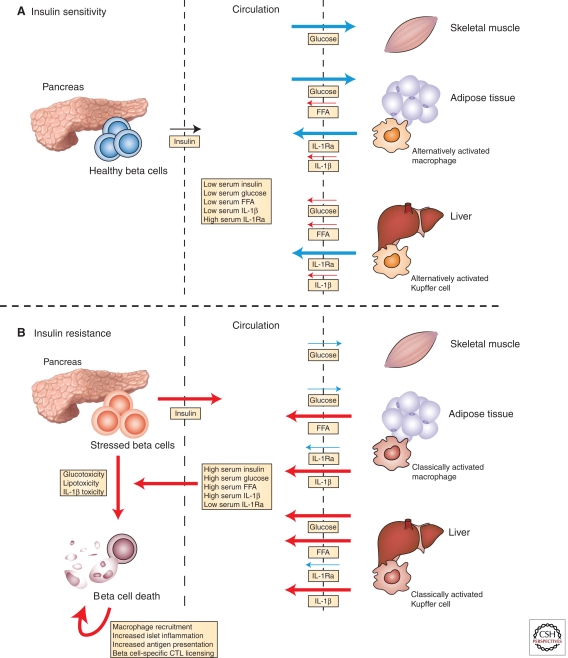
Figure 1.
Insulin resistance damages β-cells and leads to autoimmune insulitis. (A) In lean, insulin-sensitive individuals, normal insulin secretion is sufficient to induce robust uptake of glucose from the circulation by skeletal muscle and adipose tissue, to inhibit free fatty acid (FFA) release from adipose tissue, and to suppress hepatic gluconeogenesis. In such individuals, the adipose tissue macrophages and Kupffer cells have an alternative bias, resulting in expression of interleukin-1 receptor antagonist (IL1-Ra) and suppression of IL-1β. The resulting serologic state is characterized by relatively low concentrations of insulin, glucose, FFAs, and inflammatory mediators (e.g., IL-1β) and high levels of regulatory cytokines (e.g., IL-1Ra). (B) In contrast, insulin resistance abrogates insulin’s peripheral effects, resulting in reduced glucose uptake, unsuppressed hepatic gluconeogenesis, and enhanced FFA release from adipose tissue. Under these conditions, adipose tissue macrophages and Kupffer cell populations are classically activated, expressing high levels of pro-inflammatory mediators such as IL-1β, TNFα, and nitric oxide, while suppressing production of tolerogenic peptides, such as IL-10 and IL-1Ra. The serum is thus characterized by progressively higher levels of insulin, glucose, FFAs, and inflammatory mediators and reduced levels of tolerogenic peptides. Escalating insulin resistance eventually results in levels of glucose, saturated fatty acids, and inflammatory mediators that are directly toxic to β-cells (among other tissues). β-Cell damage and concomitant innate immune activation within the islet initiate β-cell-specific cytotoxic T lymphocyte (CTL) responses, which further damage the beleaguered β-cells. (Blue) Processes associated with insulin sensitivity; (red) those associated with insulin resistance. The arrow weight indicates relative flux through individual pathways.