Abstract
Mycobacterium tuberculosis infection claims approximately 2 million lives per year, and improved efficacy of the BCG vaccine remains a World Health Organization priority. Successful vaccination against M. tuberculosis requires the induction and maintenance of T cells. Targeting molecules that promote T-cell survival may therefore provide an alternative strategy to classic adjuvants. We show that the interaction between T-cell–expressed OX40 and OX40L on antigen-presenting cells is critical for effective immunity to BCG. However, because OX40L is lost rapidly from antigen-presenting cells following BCG vaccination, maintenance of OX40-expressing vaccine-activated T cells may not be optimal. Delivering an OX40L:Ig fusion protein simultaneously with BCG provided superior immunity to intravenous and aerosol M. tuberculosis challenge even 6 months after vaccination, an effect that depends on natural killer 1.1+ cells. Attenuated vaccines may therefore lack sufficient innate stimulation to maintain vaccine-specific T cells, which can be replaced by reagents binding inducible T-cell costimulators.
BCG, which is the current vaccine against Mycobacterium tuberculosis (M. tuberculosis), is effective against infantile tuberculosis meningitis and miliary tuberculosis [1] but fails to confer reliable protection against pulmonary tuberculosis in adults [2, 3]. Furthermore, tuberculosis drug treatment is lengthy and costly, and reduced compliance has led to the increase in multidrug-resistant strains [4, 5]. Because BCG confers significant protection in young children and provides cost-effective prevention from tuberculosis for developing countries, it would be desirable to modify the existing BCG vaccine to provide better protection. Indeed, many current vaccination strategies seek to improve immune priming to BCG through utilization of modified BCG strains or prime-boost strategies where an initial immune response to BCG is enhanced by a heterologous or homologous antigen boost [6, 7].
Protective immunity to M. tuberculosis requires CD4+ T cells that secrete type 1 cytokines. Although these cells are induced during natural infection, they do not ultimately limit the disease. The goal therefore is to design a vaccine that is superior to the pathogen in induction of CD4+ Th1 and CD8+ T cells. Efficient induction of acquired immunity relies on robust innate immune responses. Antigen-presenting cells play a role in priming antigen-specific T cells but must also provide sufficient stimulation to override activation-induced cell death. Similarly, cytokines produced by natural killer (NK) cells contribute to an environment conducive to CD4+ Th1 and CD8+ T-cell development and sustain later lung CD4+ T-cell effector/memory antimycobacterial responses [8–10]. Because induction of cellular responses by vaccination in humans can be difficult, pathogen epitopes or proteins are often administered with innate immune-stimulating adjuvants. In 1989 Charles Janeway referred to these adjuvants as “the immunologists’ dirty little secret” [11] because their precise composition and mode of action are not known. The use of “adjuvants” that stimulate acquired immunity directly with greater precision is therefore appealing. In addition to T-cell receptor signaling, T cells require a succession of costimulatory signals to survive. The majority of costimulatory signals required later in the activation process are induced and so make interesting targets for use as a T-cell–specific adjuvant.
OX40 (CD134, ACT 35, Tnfrsf4) is an ideal target because it is predominantly expressed on recently activated T cells but absent on naive or resting memory T cells and is found on NK cells and NK T-cell populations required for antimycobacterial immunity [12]. On naive T cells the kinetics of OX40 expression is between 12 hours and 2–3 days, depending on the T-cell stimulus [13]. On memory T cells, however, reexpression of OX40 is faster (1–4 hours) [13]. Targeting OX40 therefore provides a strategy for boosting a small proportion of relevant T cells in the context of vaccination. OX40 can act independently of any other signal to promote survival or as a costimulator with T-cell receptor signaling to drive growth and clonal expansion [14]. Three copies of OX40 bind the trimeric ligand OX40L (Tnfsf4, gp34, CD252) [15], which is induced on a number of cell types, predominantly antigen-presenting cells [12]. The criteria for expression of OX40L on the variety of cell types that express it are less clear. OX40L on antigen-presenting cells and/or B cells appears 24 hours following CD40 or Toll-like receptor ligation. Interleukin 18 (IL-18) and thymic stromal lymphopoeitin are also reported to increase OX40L expression [16, 17], and it is likely that various inflammatory cytokines impact on OX40L levels as described for tumor necrosis factor (TNF) on airway smooth muscle cells [18].
We hypothesized that ligation of OX40 during BCG-specific T-cell activation would provide superior protection upon M. tuberculosis challenge and provide a novel adjuvant that targets a relevant subset of acquired, rather than innate, immunity. We now show that a genetic deletion of OX40L causes a loss of the T-cell response to BCG, whereas OX40 ligation at the time of BCG vaccination provides enhanced protection against aerosol and intravenous M. tuberculosis challenge in a murine model compared with BCG vaccination alone. This effect is dependent on the presence of NK1.1+ cells. Our study highlights a critical point for vaccine development, namely, that the vaccine must not only prime antigen-specific T cells but also maintain the inducible costimulatory ligands on antigen-presenting cells for a sufficient length of time to allow their progression into the memory T-cell pool.
MATERIALS AND METHODS
Ethics Statement
This study was carried out in accordance with the recommendations in the Guide for the Use of Laboratory Animals of Imperial College London. All animal procedures and care conformed strictly to the UK Home Office Guidelines under the Animals (Scientific Procedures) Act 1986, and the protocols were approved by the Home Office of Great Britain (license no. 70/6646). All surgeries were performed under anesthesia, and all efforts were made to minimize suffering with a strict implementation of the replacement, reduction, and refinement principles.
Mice
Six- to eight-week-old female C57BL/6 mice were purchased from Harlan Olac Ltd. OX40L−/− mice, on a C57BL/6 background, were a gift from Kazuo Sagamura (Tohoku University School, Sendai, Japan). These mice do not harbor any gross abnormalities or apparent immunological defects when unchallenged (data not shown). All mice were kept in pathogen-free conditions according to Home Office guidelines, maintained in biosafety level 3 facilities, and given sterile water, mouse chow, and bedding.
BCG and M. tuberculosis
BCG Pasteur (Pasteur Institute, Paris, France) and M. tuberculosis H37Rv were grown in either Middlebrook 7H9 medium (Difco), supplemented with 10% albumin-dextrose-catalase (ADC; Difco), 0.2% glycerol, and 0.05% Tween 80 or on Middlebrook 7H11 agar plates supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC; Difco), 0.2% glycerol, and 10 μg/mL amphotericin B. Bacteria for infection were obtained from late logarithmic growth phase cultures. The number of bacteria was assessed by measurement of optical density (OD) at 600 nm, where an OD of 1 represented 108 colony-forming units (CFU)/mL.
Mouse Infections and Treatment
C57BL/6 and OX40L−/− mice were anesthetized with isoflourane and subcutaneously infected with 2 × 107 CFU of BCG Pasteur (in 200 μL). In some experiments BCG-infected mice were challenged intravenously (5 × 105 CFU/mL, in 200 μL) or via an aerosol route (50–100 bacilli per mouse) with M. tuberculosis H37Rv at different times after BCG infection, as noted in the text. Some groups of mice were injected intraperitoneally with 100 μg (in 200 μL) OX40L:murine immunoglobulin (Ig) G1 fusion protein (OX40L:Ig) constructed as previously described [19] on day 0. NK cell depletion was via intraperitoneal administration of 100 μg anti-NK1.1 antibody (CD161; Clone: PK136 eBiosciences) at the time of BCG vaccination and every 3 days thereafter until day 12. Untreated groups received an analogous quantity of IgG1 (Caltag). Mice were sacrificed at various time points after BCG and M. tuberculosis infection by dislocation of the cervical vertebrae. The spleens and inguinal lymph nodes (ILNs) of BCG-infected mice and the spleens and lungs of M. tuberculosis-infected mice were removed under aseptic conditions. Tissue was disrupted to a single cell suspension by passage through a 100-μM sieve (BD Labware) and aliquots plated on Middlebrook 7H11 agar plates supplemented with 10% OADC, 0.2% glycerol, and 10 μg/mL amphotericin B for CFU quantification. Red blood cells were lysed by resuspending pellets in ACK buffer (0.15 M ammonium chloride, 1 M potassium hydrogen carbonate, and 0.01 mM ethylenediaminetetraacetic acid; pH 7.2). Cell viability was assessed by trypan blue exclusion and cells resuspended in Roswell Park Memorial Institute 1640 medium containing 10% fetal calf serum, 2 mM/mL L-glutamine, 50 μg/mL penicillin, and 50 μg/mL streptomycin (R10F) at 106 cells/mL.
Flow Cytometric Analysis
Cells were stained for surface markers and analyzed by flow cytometry. All antibodies were purchased from BD Pharmingen, except anti-OX40-FITC (fluorescein isothiocyanate) (Serotec UK). In brief, 1 × 106 cells were stained using anti-CD4-Allophycocyanin (APC) and anti-CD8-PerCP. Cells were also stained with combinations of FITC-labeled antibodies specific for CD44, OX40, DX5, and PE-labeled anti-CD45RB or -CD44. Some cells were also stained with anti-CD11c-FITC, anti-CD11b-PE, and anti-OX40L-biotin, followed by streptavidin-APC (Caltag). All antibodies were diluted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA)/0.05% sodium azide (PBA). Cells were stained for 30 minutes on ice, washed with PBA, and spun for 5 minutes at 1200 rpm. After washing, cells were then fixed for 20 minutes at room temperature with 2% formaldehyde/PBS. Cells were then washed in PBA and data were acquired on a FACSCalibur, and 30 000 lymphocyte events were analyzed with CellQuest Pro software (BD Biosciences).
Enzyme-Linked Immunosorbent Spot Assays
Sterile filter plates (Millipore) were coated with anti-interferon (IFN)-γ antibodies (Pharmingen International) in 0.1M carbonate/bicarbonate buffer at pH 9.6 overnight before washing and blocking with complete medium. Then 5 × 105 cells were added to wells with four 1:2 serial dilutions. After stimulation with purified protein derivative (PPD-RT46, Statens Serum Institut), cells were cultured for 48 hours. The cells were removed by washing and the site of cytokine production detected by biotin-labeled rat antimurine IFN-γ monoclonal antibody, using BCIP/NBT (Sigma-Aldrich) as an alkaline phosphatase substrate. Spot-forming colonies were counted using a dissecting microscope and wells without PPD subtracted as background.
Proliferation Assays
For the proliferation assays, 2 × 105 cells were added to wells with four 1:2 serial dilutions. Cells were stimulated with 0.1–100 μg/mL of PPD and incubated for 24 hours. Cells were subsequently pulsed with 25 μL of tritiated thymidine (Amersham) and incubated overnight. Cells were harvested using a Beta plate counter (Wallac).
Cytokine Release Assays
For the cytokine release assays, 2 × 105 cells were stimulated with 100 μg/well of PPD and incubated for 48 hours. Supernatants were removed and the concentrations of IFN-γ and TNF-α in supernatants were quantified using OptEIA kits (Pharmingen) according to manufacturer’s instructions.
Statistical Analysis
Statistical significance was calculated using an unpaired Mann–Whitney test and Prism software. All P values of ≤.05 and ≤.01 were considered significant and are referred to as such in the text.
RESULTS
Loss of OX40L Limits Cellular Immunity to BCG
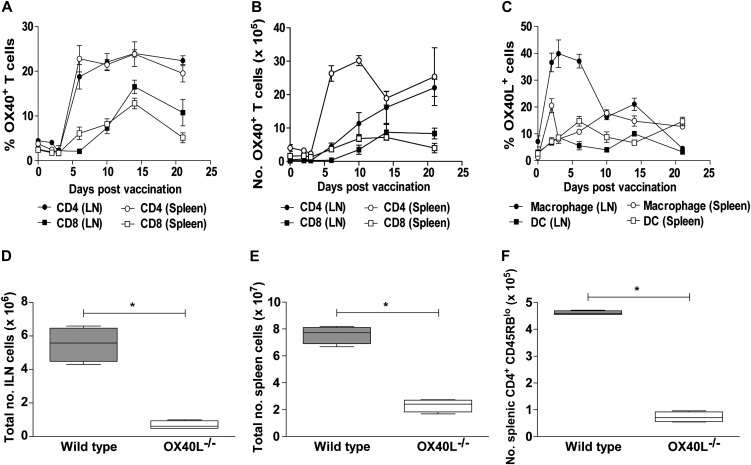
Administration of BCG subcutaneously to C57BL/6 mice led to a rapid increase of OX40 expression on CD4+ and CD8+ T cells in the ILN and spleen (Figure 1A) that was maintained for prolonged periods. Consequently, the number of OX40+ T cells was also significantly elevated after BCG vaccination (Figure 1B) Conversely, OX40L on macrophages was only transiently expressed and low or absent on ILN and spleen dendritic cells (Figure 1C). To verify the importance of OX40L in the immune response to BCG, BCG was administered subcutaneously to wild-type C57BL/6 and OX40L-/- mice. Naive OX40L-/- mice have no gross abnormalities and comparable cell numbers to wild-type mice in the spleen and ILN (data not shown). However, a targeted deletion of OX40L resulted in strikingly reduced total cell numbers in the ILN (Figure 1D) and spleen (Figure 1E) compared with wild-type C57BL/6 controls at 14 days after BCG administration, owing to reduced T-cell numbers. Activation of splenic CD4+ (Figure 1F) and CD8+ (data not shown) T cells was also reduced. Thus it would appear that in the absence of OX40L, there is inefficient priming of the T-cell response to BCG.
Figure 1.
The role of OX40/OX40L in immunity to BCG. Wild-type C57BL/6 mice were inoculated subcutaneously with 2 × 107 BCG. At defined time points, the total number of viable cells in the inguinal lymph nodes (ILNs) and spleen was enumerated by trypan blue exclusion. The percentage of OX40 expression on CD4+ and CD8+ T cells in ILNs and spleen on the days shown during BCG vaccination was evaluated by flow cytometry (A). Total number of OX40+ T cells was subsequently determined from total viable cell counts (B). OX40L on CD11b+ macrophages and CD11c+ dendritic cells (DCs) (C) was also evaluated by flow cytometry in ILNs and spleen on the days shown during BCG vaccination. Five wild-type C57BL/6 (WT, closed bars) or OX40L−/− (open bars) mice were inoculated subcutaneously with 2 × 107 BCG. Fourteen days later the ILNs (D) and spleen (E) were removed and total viable cells enumerated by trypan blue exclusion. Activated CD4+ T cells in the spleen (F) were determined by flow cytometry, identified by low-level expression of CD45RB. All graphs show results for 5 mice per group and are representative of 2–3 independent experiments. *P < .05, **P < .01 by Mann–Whitney test.
Ligation of OX40 In Vivo Enhances Th1 CD4+ and CD8+ Responses to BCG
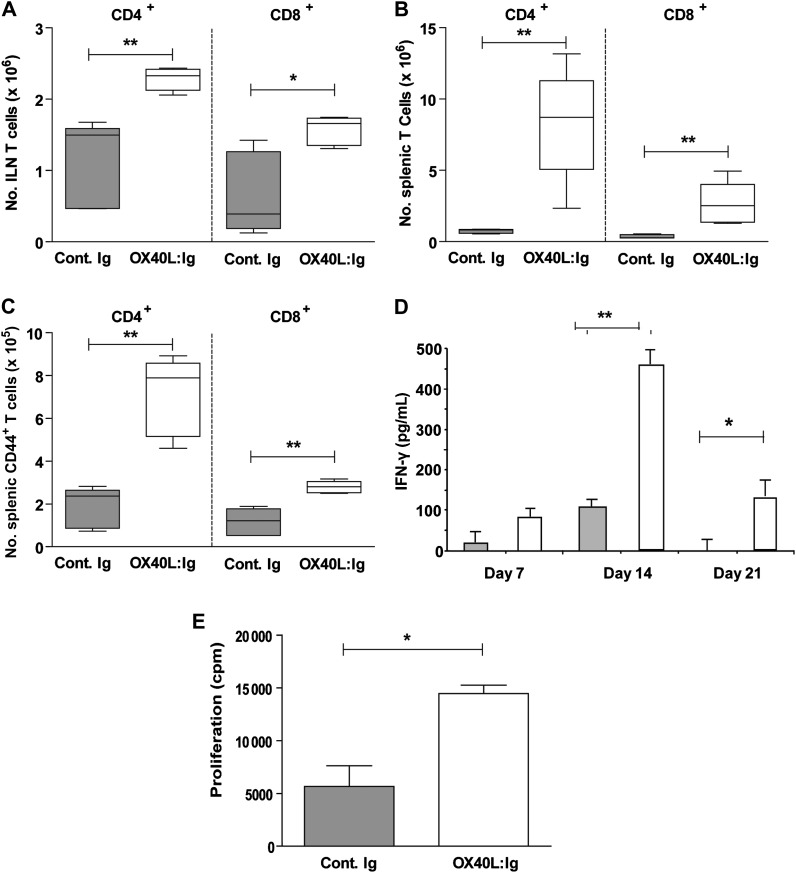
We next tested whether administration of an OX40L:Ig fusion protein to wild-type mice acted as an adjuvant to the BCG. OX40L:Ig was delivered intraperitoneally at the same time as BCG was delivered subcutaneously; the elicited immune response was evaluated at days 7, 14, and 21. CD4+ and CD8+ T cells in the ILN (Figure 2A) and spleen (Figure 2B) and their level of activation (for spleen, see Figure 2C) were significantly elevated in mice administered BCG and OX40L:Ig relative to BCG alone at days 7, 14, and 21. Results are shown for day 14 only. To analyze the influence of OX40L:Ig-adjuvanted BCG on cytokine secretion, supernatants from splenocytes taken from immunized mice and stimulated ex vivo with PPD for 48 hours were analyzed using enzyme-linked immunosorbent assay. IFN-γ (Figure 2D) release from splenocytes derived from BCG/OX40L:Ig-treated mice was elevated compared with splenocytes from control mice given BCG alone. Furthermore, proliferation of splenocytes from day 14 of treatment with BCG/OX40L:Ig in treated mice stimulated ex vivo with PPD was significantly elevated (Figure 2E).
Figure 2.
Ligation of OX40 augments immunity to BCG vaccine. C57BL/6 mice were inoculated subcutaneously with 2 × 107 BCG and administered intraperitoneal control immunoglobulin (Ig; closed bars) or OX40L:Ig (open bars). Fourteen days after BCG vaccination, the total number of cells in the inguinal lymph nodes (ILNs) and spleen was determined by trypan blue exclusion and the number of CD4+ and CD8+ cells in the ILNs (A) and spleen (B) was subsequently calculated from the percentage positive for each population by flow cytometry. The number of activated T-cell subsets in the spleen was determined from the percentage of T cells expressing CD44 as adjudged by flow cytometry at day 14 of BCG/OX40L:Ig or BCG-alone treatment (C). Interferon (IFN)–γ release from splenocytes (D, mean ± SD) and proliferation of splenocytes from day 14 (E) after treatment with BCG/OX40L:Ig or BCG alone was determined in response to ex vivo purified protein derivative (100 μg/mL). All graphs show results for 5 mice per group and are representative of 2–3 independent experiments. *P < .05, **P < .01 by Mann–Whitney test.
Coadministration of OX40L:Ig With BCG Vaccine Augments Protection to Subsequent M. tuberculosis Challenge
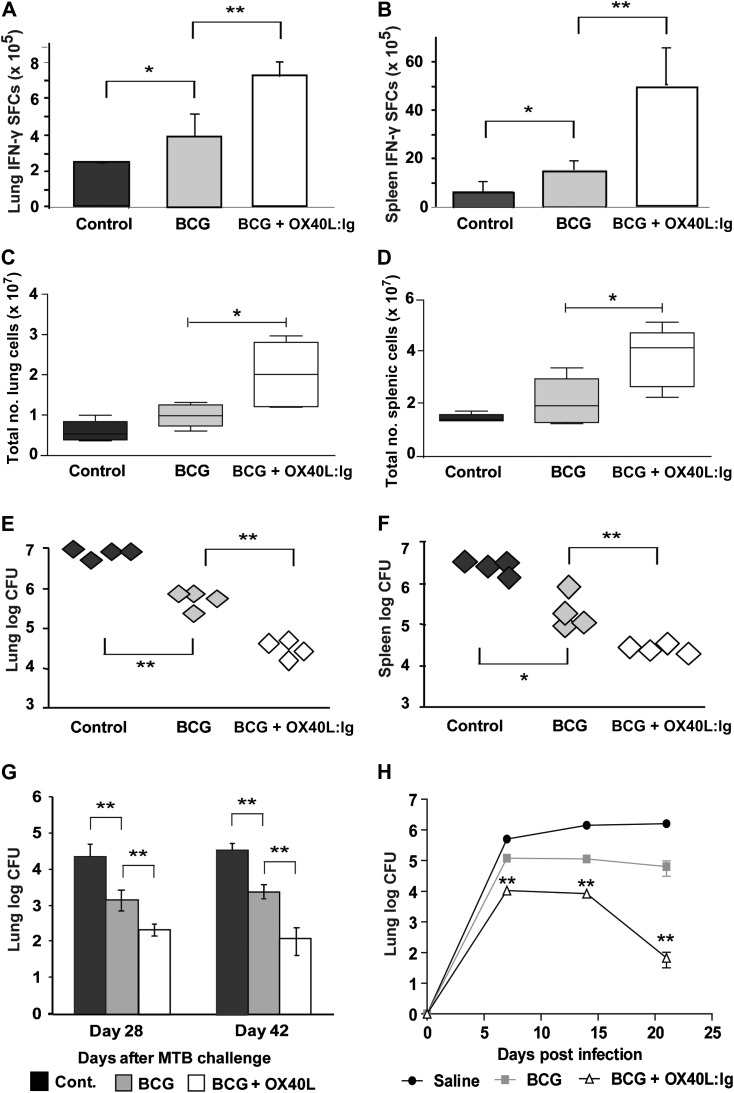
To test the implications of enhanced T-cell responses and Th1-associated cytokines, vaccinated and control mice were challenged by intravenous injection of 5 × 105 virulent M. tuberculosis 42 days after initial vaccination. Fourteen days after M. tuberculosis challenge, soluble Th1 cytokine production by lung and splenic cells after 48 hours of incubation with PPD was elevated in M. tuberculosis-challenged mice previously vaccinated with BCG and OX40L compared with those vaccinated with BCG alone or mock vaccinated controls (data not shown). Enzyme-linked immunosorbent spot analysis of T cells from the same mice revealed them to be a dominant source of IFN-γ (Figure 3A and 3B) and TNF. Total number of cells in the lung (Figure 3C) and spleen (Figure 3D) was also significantly raised by combining BCG with OX40L:Ig on day 14 after M. tuberculosis challenge compared with the control groups. The number of CFUs recovered after M. tuberculosis challenge from the lungs (Figure 3E) and spleen (Figure 3F) of BCG-vaccinated mice was reduced by approximately 1 log compared with mock-vaccinated controls at 14 days after challenge. A further 1-log reduction over and above that of BCG alone was observed in organs from mice that received BCG along with the OX40L:Ig adjuvant (Figure 3E and 3F).
Figure 3.
The role of OX40/OX40L in immunity to BCG and Mycobacterium tuberculosis (M. tuberculosis) challenge. Five mice per group were inoculated subcutaneously with phosphate-buffered saline (cont.), BCG plus control immunoglobulin (Ig) (BCG), or BCG + OX40L:Ig and challenged with 5 × 105 M. tuberculosis (strain R37rv) intravenously or 50–100 bacilli by aerosol. In mice intravenously challenged 42 days after vaccination, interferon (IFN)–γ spot-forming colonies (SFCs) from purified protein derivative –stimulated lung cells (A) or splenocytes (B) was enumerated by enzyme-linked immunosorbent spot assay (mean + SD of 5 mice per group) 14 days after challenge. In the same mice, total numbers of viable cells (C and D) and colony-forming units (CFUs) (E and F) in the lung (C and E) and spleen (D and F) 14 days after M. tuberculosis challenge were determined. In aerosol-challenged mice (G and H), the number of M. tuberculosis CFUs was determined by diluting homogenates of lung in mice challenged with M. tuberculosis 28 days (G) or 180 days (H) after BCG vaccination. All graphs show results for 4–5 mice per group and are representative of 2–3 independent experiments. *P < .05, **P < .01 by Mann–Whitney test (in H, significance is presented for BCG vs BCG + OX40L:Ig).
To mimic the more natural route of infection with M. tuberculosis, we next investigated whether BCG administered with an OX40L:Ig adjuvant conferred greater protection against aerosolized M. tuberculosis. C57BL/6 mice sham treated or vaccinated with BCG alone or BCG and OX40L:Ig were challenged after 28 days with M. tuberculosis H37Rv via the aerosol route using a concentration of bacteria that provides approximately 50–100 bacteria per total lung. Protection was assessed 4 and 6 weeks later. We chose these later time points after challenge to show long-term protection using our adjuvanted strategy. As with intravenous challenge with M. tuberculosis, the number of CFUs recovered after M. tuberculosis challenge from the lungs (Figure 3G) and spleen (data not shown) of BCG-vaccinated mice was reduced by approximately 1 log compared with mock vaccinated controls at 4 and 6 weeks after challenge. A further 1-log reduction over and above that of BCG alone was observed in organs from mice that received BCG along with the OX40L:Ig adjuvant at both time points (Figure 3G). This further limitation of M. tuberculosis was also apparent when the interval between vaccination and challenge was extended to 180 days and CFUs were analyzed in an early time course up to the sampling points shown for the intravenous M. tuberculosis route (Figure 3H).
Importance of NK1.1+ Cell Responses to Superior Protection in OX40L:Ig-Treated BCG-Vaccinated Mice
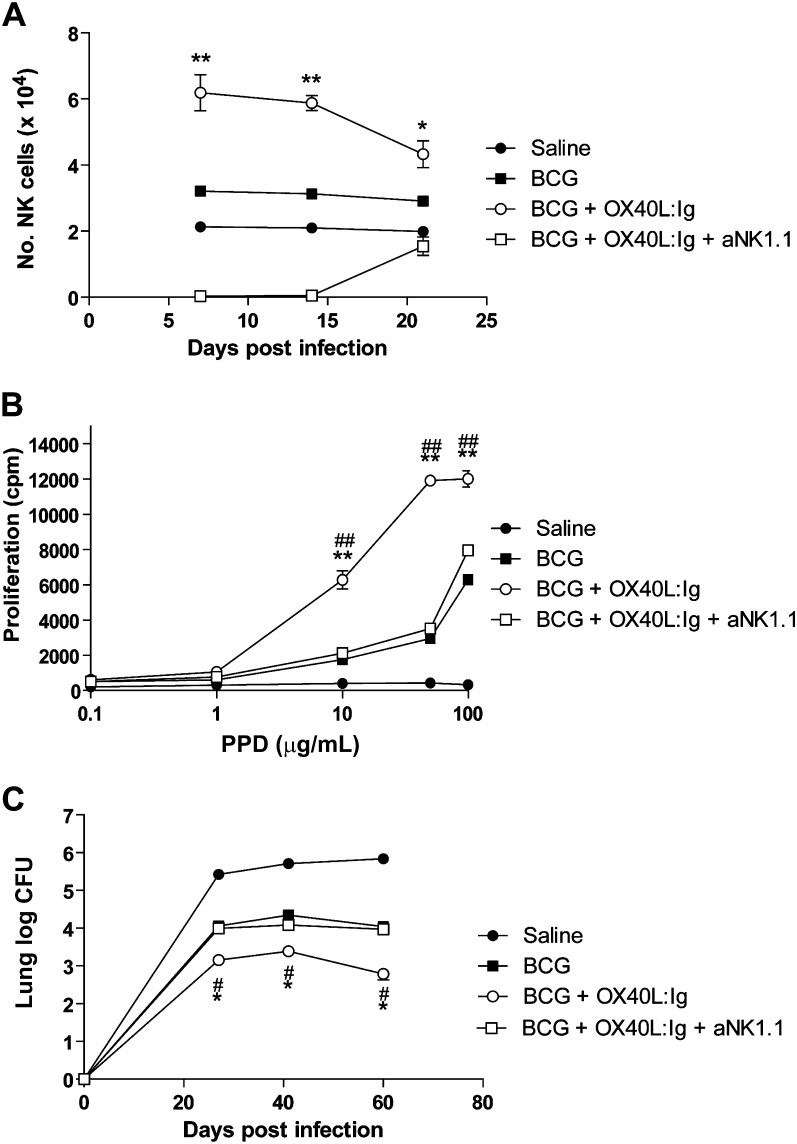
Because OX40 is expressed on NK1.1+ cells and its ligation enhances the development of Th1 responses, we assessed their importance in augmented immunity to BCG vaccine and subsequent protection from M. tuberculosis. Administration of BCG subcutaneously to C57BL/6 mice led to enhanced NK cells (DX5+CD4-CD8-) in the spleen relative to saline control mice at days 7, 14, and 21 after vaccination (Figure 4A). Coadministration of intraperitoneal OX40L:Ig at time of vaccination resulted in an increase in NK cell numbers above that of BCG alone (Figure 4A). Administration of an anti-NK1.1−depleting antibody at time of vaccination, and every 3 days thereafter until day 12 after vaccination, abolished NK cells at days 7 and 14, but numbers were restored to baseline levels by day 21 (Figure 4A). The significance of the enhanced NK cell response in OX40L:Ig-treated mice to development of T-cell immunity was demonstrated by the enhanced spleen cell proliferation to PPD relative to saline or BCG-alone treated mice (Figure 4B). Furthermore, the enhanced protection achieved by combining BCG vaccination with OX40L:Ig on intravenous M. tuberculosis was abolished when NK1.1+ cells were depleted at time of vaccination (Figure 4C).
Figure 4.
A critical role for natural killer (NK) cells in OX40L:Ig-mediated protection from Mycobacterium tuberculosis (M. tuberculosis). C57BL/6 mice were inoculated subcutaneously with saline (closed circle) or 2 × 107 BCG coadministered with intraperitoneal Control Ig (closed square), OX40L:Ig (open circle) or OX40L:Ig/anti-NK1.1 (open square). NK cells were examined by flow cytometry (CD4−CD8−DX5+) in the spleen at indicated time points after BCG vaccination (A). At 14 days after BCG vaccination, proliferative responses of spleen cells from each group were adjudged by H-3-thymidine incorporation in response to purified protein derivative (PPD) (B). (C) Groups of mice were challenged with intravenous injection 6 weeks after BCG administration and the number of colony-forming units (CFU) of M. tuberculosis was determined by diluting homogenates of lung at 25, 40, and 60 days after challenge. All graphs show results for 5–6 mice per group and are representative of 2–3 independent experiments. *P < .05, **P < .01 by Mann–Whitney U test for BCG vs BCG + OX40L:Ig. #P < .05, ##P < .01 by Mann–Whitney U test for BCG + OX40L:Ig vs BCG + OX40L:Ig + anti-NK1.1.
DISCUSSION
We show that in the absence of OX40L, immunity to BCG vaccination is severely impaired, unlike the dispensable role of another TNF superfamily member, LIGHT [20]. Furthermore, provision of an OX40 agonist at the time of BCG vaccination affords superior immunity and subsequently better control of M. tuberculosis compared with BCG vaccination alone. We are the first to demonstrate the adjuvant potential of OX40 agonists for mycobacterial disease, whereas efficacy is also documented for hepatitis B surface antigen [21], Simian immunodeficiency virus [22], murine cytomegalovirus [23], and, in conjunction with anti-cytotoxic T lymphocyte antigen (CTLA)–4, Leishmania donovani [24]. As in our study, OX40 agonists result in a Th1-cytokine–dominated response. OX40 ligation increases interleukin 2 (IL-2) production from effector T cells [13] and IL-2Rα, which increases IFN-γ in environments rich in interleukin 12 (IL-12) and IL-18 [25, 26]. OX40 agonists also increase IL-12R expression and prevent the expression of CTLA-4, FOXp3, and interleukin 10 (IL-10) [27–30].
Multiple studies have attempted to improve the existing BCG vaccine through the generation of recombinant BCG (rBCG) strains or coadministration of the parental BCG strain with immunological adjuvants (for reviews, see [31, 32] and references therein). The first recombinant vaccine with greater efficacy in animal models than the parental BCG strain overexpresses Ag85B of M. tuberculosis and confers up to a 1-log reduction in M. tuberculosis CFUs. In a phase 1 trial in humans, this vaccine enhanced antigen-specific T-cell proliferation and cytokine production. There has been a concerted effort to develop further recombinant BCG vaccines that overexpress members of the Ag85 complex either alone or associated with other M. tuberculosis antigens or recombinant cytokines. However, only occasionally do they exhibit any improvement to parental BCG in conferring protection to M. tuberculosis. Other notable efforts to develop superior rBCG vaccines are overexpression of a pore-forming cytolysin, lysteriolysin, from Listeria monocytogenes; reintroduction of selected genes lost during attenuation of BCG [33]; overexpression of mycobacterial latency associated antigens [34–37]; and expression of functional cytokines alone or in combination with mycobacterial antigens [38, 39]. Again, efficacy beyond the conventional BCG in conferring protection to tuberculosis is either unknown or limited. Innate immune molecules have been targeted as adjuvants to BCG vaccine. For example, IL-12, TNF, lactoferrin, and oligodeoxynucleotides containing CpG motifs enhance Th1 responses to BCG and, in some instances, reduce subsequent M. tuberculosis burden [40–43]. Our current strategy to modulate OX40 signaling confers between a 1- and 4-log reduction in M. tuberculosis burden of challenged mice compared with BCG alone. This dramatic enhancement in protection is superior to the vast majority of previous manipulations of BCG and is a promising strategy to improve protection against M. tuberculosis.
We must consider that OX40 is also expressed on other cell types and that OX40L:Ig may promote their function. NK cells produce excess IFN-γ when cocultured with OX40L-expressing dendritic cells. Their enhancement in OX40L:Ig-adjuvanted BCG-vaccinated mice that we report here is therefore likely to facilitate Th1 T-cell development [44]. Indeed, the superior protection to M. tuberculosis observed in OX40L:Ig-treated mice was abolished with NK1.1+ cell depletion during BCG priming, suggesting a critical role for this cell type in OX40-dependent promotion of Th1 immunity. Despite the phagosomal location of both BCG and M. tuberculosis, CD8+ T-cell priming is also an important component of protective immunity and is thought to arise through cytosolic processing of M. tuberculosis antigens [45]. Though originally thought not to impact on CD8+ T cells, OX40 is now recognized as promoting their activity either directly or indirectly via CD4+ T-cell help [12, 46]. The enhancement of activated CD8+ T cells in our study may therefore reflect direct activation by OX40L:Ig or additional help from the expanded CD4+ T-cell population.
One question that arises in proposing OX40 agonists as vaccine adjuvants is that if OX40:OX40L interactions are beneficial, why doesn’t endogenous OX40L on antigen-presenting cells suffice? We believe it is the length of time OX40L persists on APCs that determines the strength of the T-cell response. BCG causes downregulation of OX40L expression on thymic stromal lymphopoietin–stimulated dendritic cells in vitro [47]. Furthermore, mild infections (such as BCG) and attenuated, heat-inactivated or peptide-based vaccines may not provide a sufficiently robust innate stimulus to maintain OX40L expression. Optimal T-cell expansion and memory development requires 3 distinct signals: (1) antigen, (2) lipopolysaccharide or equivalent danger signal, and (3) ligation of OX40 [48]. The latter is clearly not maintained in response to BCG. This places an emphasis for successful vaccination on the cell presenting the vaccine.
In summary, we argue that BCG vaccination fails to maintain OX40L expression on APCs and thus does not maintain vaccine-activated T cells. Through ligation of OX40 with an OX40L:Ig fusion protein, we selectively boost recently activated mycobacterium-specific T-cell proliferation and Th1 cytokine production and development of an augmented memory response that is significantly superior to BCG in conferring protection to both intravenous and aerosol M. tuberculosis infection. Intriguingly, NK 1.1 + cells appear to be critical in the OX40-mediated augmentation of immunity and protection, most likely through release of IFN-γ following engagement of OX40 on their surface and ensuing promotion of a Th1 immune response. Our results imply that sufficient innate stimulation is as important as T-cell priming in vaccine efficacy and that addition of molecules specific for late T-cell costimulators provides a targeted approach to overcome an attenuated response.
Notes
Financial support.
This work was supported by the Medical Research Council (G0802752 to T. H.) and The Wellcome Trust (071265/Z/03/Z to M. C., 082727/Z/07/Z and 095707/Z/11/Z to R. S.).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 2.Svenson S, Kallenius G, Pawlowski A, Hamasur B. Towards new tuberculosis vaccines. Hum Vaccin. 2010;6:309–17. doi: 10.4161/hv.6.4.10711. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.van Rie A, Warren R, Richardson M, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–9. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann SH. Envisioning future strategies for vaccination against tuberculosis. Nat Rev Immunol. 2006;6:699–704. doi: 10.1038/nri1920. [DOI] [PubMed] [Google Scholar]
- 6.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–76. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- 7.Evans JH, Horowitz A, Mehrabi M, et al. A distinct subset of human NK cells expressing HLA-DR expand in response to IL-2 and can aid immune responses to BCG. Eur J Immunol. 2011;41:1924–33. doi: 10.1002/eji.201041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolberg VR, Chiu BC, Schmidt BM, Kunkel SL, Sandor M, Chensue SW. CC chemokine receptor 4 contributes to innate NK and chronic stage T helper cell recall responses during Mycobacterium bovis infection. Am J Pathol. 2011;178:233–44. doi: 10.1016/j.ajpath.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall LJ, Clare S, Dougan G. NK cells influence both innate and adaptive immune responses after mucosal immunization with antigen and mucosal adjuvant. J Immunol. 2010;184:4327–37. doi: 10.4049/jimmunol.0903357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 1:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–7. [PubMed] [Google Scholar]
- 13.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–5. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 14.Compaan DM, Hymowitz SG. The crystal structure of the costimulatory OX40-OX40L complex. Structure. 2006;14:1321–30. doi: 10.1016/j.str.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maxwell JR, Yadav R, Rossi RJ, et al. IL-18 bridges innate and adaptive immunity through IFN-gamma and the CD134 pathway. J Immunol. 2006;177:234–45. doi: 10.4049/jimmunol.177.1.234. [DOI] [PubMed] [Google Scholar]
- 18.Krimmer DI, Loseli M, Hughes JM, et al. CD40 and OX40 ligand are differentially regulated on asthmatic airway smooth muscle. Allergy. 2009;64:1074–82. doi: 10.1111/j.1398-9995.2009.01959.x. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–9. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 20.Musicki K, Briscoe H, Britton WJ, Saunders BM. LIGHT contributes to early but not late control of Mycobacterium tuberculosis infection. Int Immunol. 2010;22:353–8. doi: 10.1093/intimm/dxq013. [DOI] [PubMed] [Google Scholar]
- 21.Du X, Zheng G, Jin H, et al. The adjuvant effects of co-stimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J Gene Med. 2007;9:136–46. doi: 10.1002/jgm.1004. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg AD, Thalhofer C, Morris N, et al. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–85. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys IR, Loewendorf A, de TC, et al. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T Cells: A CD4-dependent mechanism. J Immunol. 2007;179:2195–202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 24.Zubairi S, Sanos SL, Hill S, Kaye PM. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. Eur J Immunol. 2004;34:1433–40. doi: 10.1002/eji.200324021. [DOI] [PubMed] [Google Scholar]
- 25.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–43. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 26.Williams CA, Murray SE, Weinberg AD, Parker DC. OX40-mediated differentiation to effector function requires IL-2 receptor signaling but not CD28, CD40, IL-12Rbeta2, or T-bet. J Immunol. 2007;178:7694–702. doi: 10.4049/jimmunol.178.12.7694. [DOI] [PubMed] [Google Scholar]
- 27.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–8. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 28.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–30. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 29.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–10. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Xiao X, Demirci G, Li XC. OX40 controls islet allograft tolerance in CD154 deficient mice by regulating FOXP3+ Tregs. Transplantation. 2008;85:1659–62. doi: 10.1097/TP.0b013e3181726987. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann SH. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity. 2010;33:567–77. doi: 10.1016/j.immuni.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 32. Bastos RG, Borsuk S, Seixas FK, Dellagostin OA. Recombinant Mycobacterium bovis BCG. [DOI] [PubMed]
- 33.Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reece ST, Nasser-Eddine A, Dietrich J, et al. Improved long-term protection against Mycobacterium tuberculosis Beijing/W in mice after intra-dermal inoculation of recombinant BCG expressing latency associated antigens. Vaccine. 2011;29:8740–4. doi: 10.1016/j.vaccine.2011.07.144. [DOI] [PubMed] [Google Scholar]
- 35.Speranza V, Colone A, Cicconi R, et al. Recombinant BCG-Rv1767 amount determines, in vivo, antigen-specific T cells location, frequency, and protective outcome. Microb Pathog. 2010;48:150–9. doi: 10.1016/j.micpath.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Shi C, Chen L, Chen Z, et al. Enhanced protection against tuberculosis by vaccination with recombinant BCG over-expressing HspX protein. Vaccine. 2010;28:5237–44. doi: 10.1016/j.vaccine.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 37.Nolan ST, Lamichhane G. Protective efficacy of BCG overexpressing an L, D-transpeptidase against M. tuberculosis infection. PLoS ONE. 2010;5:e13773. doi: 10.1371/journal.pone.0013773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray PJ, Aldovini A, Young RA. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc Natl Acad Sci U S A. 1996;93:934–9. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng Y, Bao L, Yang X. Evaluation of immunogenicity and protective efficacy against Mycobacterium tuberculosis infection elicited by recombinant Mycobacterium bovis BCG expressing human Interleukin-12p70 and Early Secretory Antigen Target-6 fusion protein. Microbiol Immunol. 2011;55:798–808. doi: 10.1111/j.1348-0421.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- 40.Freidag BL, Melton GB, Collins F, et al. CpG oligodeoxynucleotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect Immun. 2000;68:2948–53. doi: 10.1128/iai.68.5.2948-2953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramp JC, McMurray DN, Formichella C, Jeevan A. The in vivo immunomodulatory effect of recombinant tumour necrosis factor-alpha in guinea pigs vaccinated with Mycobacterium bovis bacille Calmette-Guerin. Clin Exp Immunol. 2011;165:110–20. doi: 10.1111/j.1365-2249.2011.04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang SA, Arora R, Kruzel ML, Actor JK. Lactoferrin enhances efficacy of the BCG vaccine: comparison between two inbred mice strains (C57BL/6 and BALB/c) Tuberculosis (Edinb) 2009;89 (Suppl 1):S49–54. doi: 10.1016/S1472-9792(09)70012-5. [DOI] [PubMed] [Google Scholar]
- 43.Hwang SA, Wilk K, Kruzel ML, Actor JK. A novel recombinant human lactoferrin augments the BCG vaccine and protects alveolar integrity upon infection with Mycobacterium tuberculosis in mice. Vaccine. 2009;27:3026–34. doi: 10.1016/j.vaccine.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Lou Y, Lizee G, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–75. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grotzke JE, Siler AC, Lewinsohn DA, Lewinsohn DM. Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway. J Immunol. 2010;185:4336–43. doi: 10.4049/jimmunol.1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duttagupta PA, Boesteanu AC, Katsikis PD. Costimulation signals for memory CD8+ T cells during viral infections. Crit Rev Immunol. 2009;29:469–86. doi: 10.1615/critrevimmunol.v29.i6.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokoi T, Amakawa R, Tanijiri T, et al. Mycobacterium bovis Bacillus Calmette-Guerin suppresses inflammatory Th2 responses by inducing functional alteration of TSLP-activated dendritic cells. Int Immunol. 2008;20:1321–9. doi: 10.1093/intimm/dxn094. [DOI] [PubMed] [Google Scholar]
- 48.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–12. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]