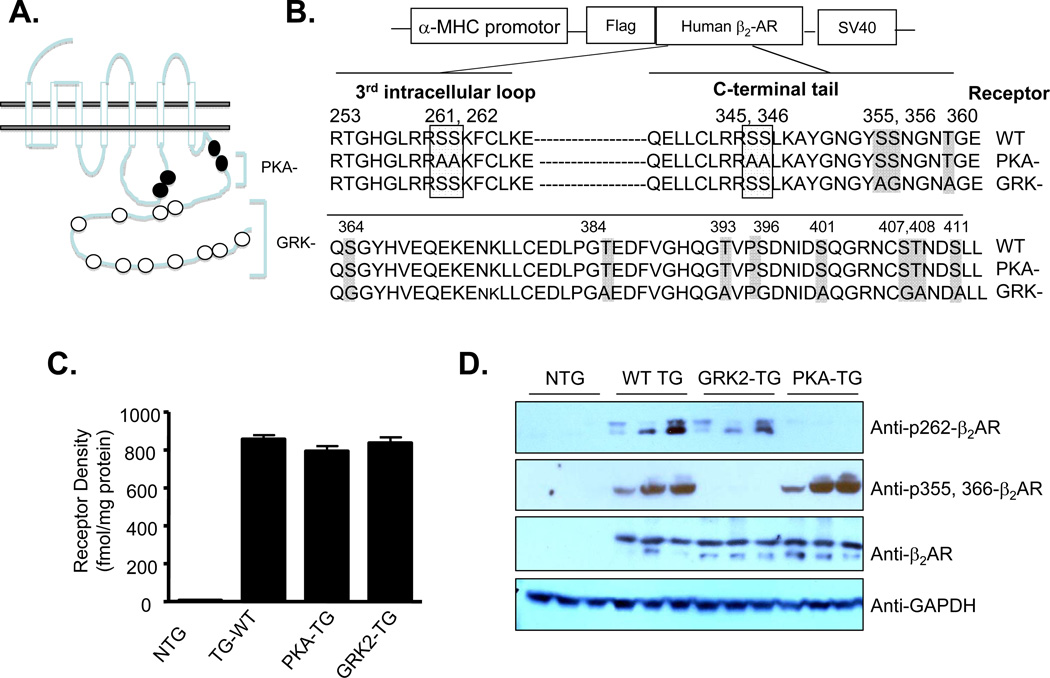
Figure 2. Transgenic mice with cardiac-specific overexpression of wild type (WT) human β2AR or its mutants lacking PKA or GRK phosphorylation sites.
(A) Schematic presentation showing β2AR phosphorylation sites for PKA or GRK. (B) The specific sequences of WT β2AR or its mutants lacking PKA (PKA-) or GRK (GRK-) phosphorylation sites. (C) The β2AR density was 9.6 ± 1.3 fmol/ mg protein and total βAR density was 28.6 ± 3.4 fmol/ mg protein in NTG mice (n=6). In transgenic mice, β2AR density was 856 ± 45, 828 ± 33 and 850 ± 23 fmol/mg protein for WT TG, PKA- TG, and GRK- TG mice, respectively, (n=6 for each group). (D) Phosphorylation of β2AR in PKA or GRK sites were assayed by Western blot using a site-specific antibody reacting with phosphorylated β2AR at a PKA site (aa262) or GRK sites (aa355 and aa366). The antibodies were raised against the peptides CDRTGHGLRRSpSKF-NH2 for the anti-pSer262 PKA site (clone 2G3) and CKAYGNGYpSpSNGN-NH2 for the anti-pS (Ser355, 356) (clone 5C3). Total expression of β2AR in transgenic mouse hearts was detected by Western blot using an antibody reacting with β2ARs.