Abstract
Chronic granulomatous disease (CGD) is a rare hereditary disease in which phagocytes have difficulty forming the superoxide radical required to kill certain pathogens. Individuals with CGD are susceptible to a specific set of infections and granulomatous lesions. We present the case of a 15 year old male with X-linked CGD who presented with unremitting cough and fevers. He had a left sided pneumonia which persisted despite home IV antibiotics. He was admitted to an outside facility for bronchoalveolar lavage to obtain cultures and polymerase chain reaction (PCR). Computed Tomography (CT) of chest, abdomen and pelvis was done for baseline evaluation of extent of disease. CT revealed a fluid collection in the prostatic fossa, later determined to be a prostatic abscess. To our knowledge, this is the first reported case of a prostatic abscess in a pediatric patient with CGD.
Keywords: Chronic granulomatous disease, CGD, prostatic abscess, pediatric patient
Introduction
Chronic granulomatous disease (CGD) is a rare inherited primary immunodeficiency initially characterized in the 1950s as “a fatal granulomatosis of childhood”(1). Individuals with CGD have a defect in the Nicotinamide Adenine Dinucleotide Phosphate-Oxidase (NADPH oxidase) enzyme complex, the role of which is to generate oxygen radicals, which go on to form hydrogen peroxide and hypohalous acid, or bleach (2). The NADPH oxidase enzyme system is composed of several subunits. The phagocyte glycosylated subunit (gp91phagocyte oxidase (phox)) and phagocyte nonglycosyated subunit (p22phox) are located in the cell membrane and form cytochrome b558, the transmembrane conduit for electrons delivered to molecular oxygen. The other three subunits, p40phox, p47phox and p67phox, are located in the cytoplasm. The beta chain of Cytochrome b (−245) (CYBB), the gene encoding gp91 phox is located on the X chromosome, whereas the genes encoding the other subunits are autosomal recessive (3). The clinical manifestations of X-linked CGD, the most severe form, are typically recognized in the first year of life and include infections and granulomatous lesions (4). The lung, skin, lymph nodes and liver are the most frequently infected sites. Abscesses are common in CGD but not in the prostate, they usually present in the liver (4,5) but have also been reported to occur at inguinal, ischiorectal, spinal, skin, perianal, deep intra-abdominal, pelvic, brain, spleen, cervical and splenic sites (5,6–7).
The inflammatory complications of CGD are most prominent in the gastrointestinal and genitourinary tracts with urinary tract involvement more common in those with defects in gp91phox or p22phox (4,6). Urological manifestations in patients with CGD are infrequently reported and the true incidence is unknown. Additionally, prostatic abscesses are uncommon and rarely diagnosed (8). They are most often associated with prostatitis, typically in elderly men with predisposing conditions, such as immunosuppression, diabetes mellitus, infra-vesical obstruction, urinary tract instrumentation or catheterization of the bladder (8). Importantly, immunosuppressant conditions may promote the formation of prostatic abscesses.
Case Report
A 15-year-old male with X-linked CGD diagnosed at 1 year old presented with complaints of cough and fever. He was diagnosed with pneumonia and admitted for treatment. After failing 6 days of inpatient treatment with vancomycin, piperacillin/tazobactam and itraconazole, his regimen was changed to voriconazole, vancomycin and piperacillin/tazobactam. He continued interferon gamma for CGD, which he received 3 times a week. CT of the chest showed left lower lobe consolidation, ground glass nodules at lung bases, right upper lobe scarring, and multiple blebs in the right mid and lower lobes. He was asymptomatic throughout the initial stay and was discharged home on piperacillin/tazobactam, oral linezolid and oral voriconazole. On follow up he reported unremitting fevers of 102F and cough. On physical exam, he was tachypneic with decreased breath sounds in the right and left lower lobes. Genitourinary (GU) exam was deferred as he denied GU symptoms. WBC count was in the reference range. Repeat CT scan showed worsening consolidation of the left lung with right sided blebbing. Due to worsening left pneumonia on exam and CT scan despite the 3 weeks of antibiotic treatment, he was readmitted.
Finding a palpable liver led to a CT of the abdomen and pelvis. Neither urinalysis nor urine culture showed any evidence of urinary tract infection (UTI). However, CT of the pelvis showed an irregular and septated cystic lesion in the prostate that was suspected to be a prostatic urethral diverticulum, utricle cyst, mullerian duct cyst or a prostatic abscess Due to suspicion of a prostate abscess, further examination was pursued. Transrectal ultrasound guided prostatic aspiration yielded 2–3mL of pus, which was sterile and had no organisms seen on Gram stain. Throughout that hospitalization he remained febrile but without urinary complaints. A left lung biopsy was non-diagnostic. He remained febrile without change in his prostatic abscess following aspiration. He was transferred to the NIH.
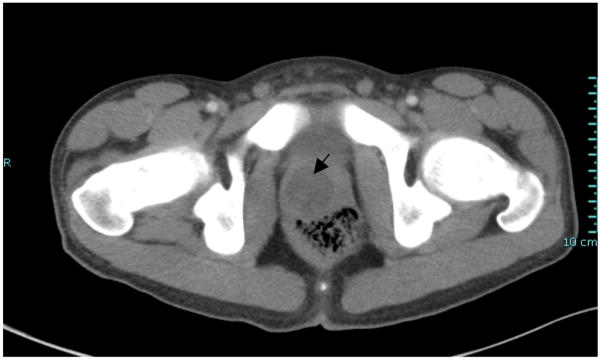
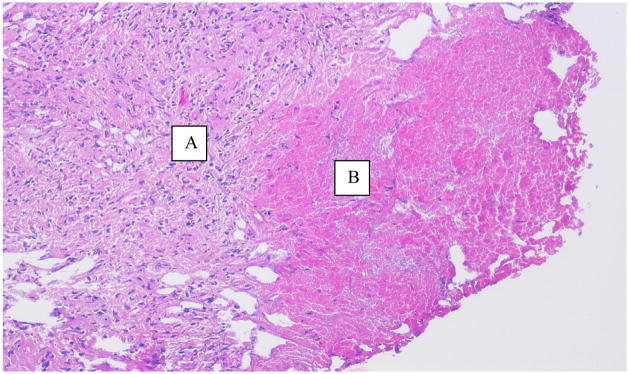
On presentation, his only complaint was a dry cough and he had no urological complaints. His fevers persisted despite meroponem, linezolid and voricanozole. On digital rectal exam, the prostate was, warm, mildly tender and enlarged with a boggy mass just right of the midline sulcus. He was febrile, tachycardic, and tachypneic with hypotension. Repeat CT showed a 3cm round abscess in an enlarged prostate (Figure 1). The patient was taken to the operating room where examination under anesthesia confirmed the pre-operative findings. Just to the right of midline posteriorly, transurethral resection (TUR) and drainage of the prostate abscess was performed. The abscess was unroofed revealing the abscess cavity below. Unlike TUR of an abscess in the elderly, classic “oozing pus” was not seen. Microbiological evaluation of the pus was performed. Wound culture, gram stain, anaerobe culture, fungal culture, wet mount, mycobacteria stain and acid fast stain were all negative except for a single colony of Candida albicans isolated from the wound culture. Prostate biopsies were performed and showed on Hematoxylin and eosin (H&E) partial effacement of the normal prostatic architecture and replacement by necrotizing granulomatous inflammation (Figure 2). Special stains with Periodic acid-Schiff (PAS), Grocott’s methenamine silver stain (GMS), Brown-Hopps (B&H) and AFB-Fite (Acid fast bacteria-fite) were negative for fungal organisms, bacteria and acid-fast bacilli. On postoperative day one, his fevers diminished and he showed marked clinical improvement. He remained afebrile after the second postoperative day.
Figure 1.
CT of abdomen done at our institution, post transrectal guided needle aspiration (at outside institution). Shows prostatic fluid abscess, about 3cm in size.
Figure 2.
H&E stain at 10× magnification showing necrotizing granulomatous inflammation in the prostate. A- Rim of granulomatous tissue, B- Necrosis
CT guided lung biopsy for persistent pneumonia yielded Burkholderia cepacia susceptible to meropenem and resistant to piperacillin/tazobactam. After one week, the patient was discharged home afebrile and much improved. On follow up at one month postoperatively, CT showed marked decreased in the prostate lesion (Figure 3).
Figure 3.
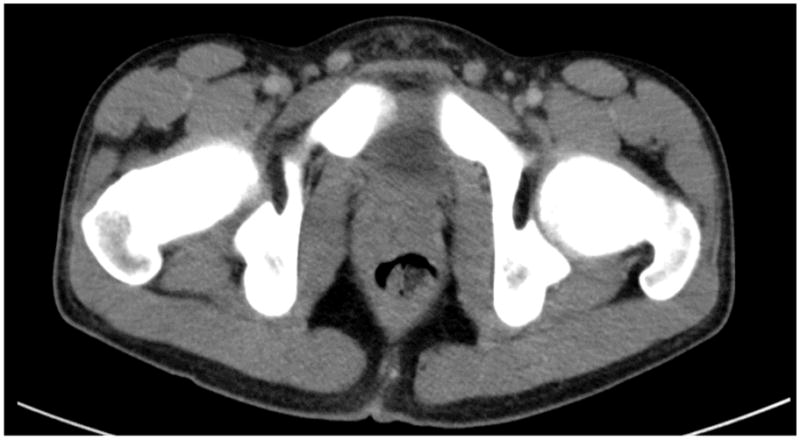
CT of abdomen 2 weeks post TUR of prostatic abscess, showing marked decrease in prostate lesion and disappearance of low attenuation lesion consistent with resolving abscess.
Discussion
Reported genitourinary involvement in patients with CGD includes bladder granulomata, ureteral obstruction and urinary tract infection (1). In addition, there have been isolated reports of the occurrence of pseudotumors and tumors of the bladder, eosinophilic cystitis, end stage renal failure, immune complex and chronic glomerulonephritis, prostatic abscess in an adult patient, xanthrogranulomatous pyelonephritis, and renal amyloidosis in patients with CGD (9,10–19). A recent series in Europe revealed a 23% occurrence of kidney or urinary tract involvement with infection being the most common manifestation. An early series revealed a 38% occurrence of urological manifestations in patients with CGD; 20% had a history of urinary tract infection, 15% had a granuloma or stricture in the urinary tract and 12% had abnormal renal function with an elevated creatinine level and 17% had other manifestations. Fifty percent of patients in that series with genitourinary manifestations were X-linked, while only 27% of patients had autosomal recessive inheritance (1). However, to date, prostatic involvement has not been reported in a pediatric patient with CGD. In addition, the occurrence of a prostate abscess in the pediatric population is rare and has been reported few times in the literature. The few reports on prostatic abscesses in the pediatric population are mostly focused on newborns. Furthermore, there are only isolated reports and no consensual review. Prostatic abscesses in pediatric patients are predominantly metastatic prostatic abscesses (MPA) (20).
The microbiologic etiology of this patient’s abscess is undetermined. However, the histopathology showed necrosis and granulomatous changes, which are clear signs of infection. His clinical course was complex and he had a common cause of pneumonia in CGD, Burkholderia cepacia. The likelihood that the prostate abscess was indeed the cause of his fever and impending sepsis is inferred from the resolution of the fever and significant clinical improvement almost immediately after the resection of the prostatic abscess. In this case, a single colony of Candida that grew from the prostatic abscess is of indeterminate significance and while it’s puzzling that the urine and wound culture were both negative in our patient, it’s not uncommon. Pre-existing antibiotic therapy often makes the identification of the pathogen impossible, especially when midstream urine is analyzed (21). A recent series examined 18 patients with prostatic abscesses and 6 of those patients had sterile urine cultures, those 6 patients were all receiving pre-existing antibiotic therapy. That is particularly interesting and similar to our patient who also had a sterile urine culture in addition to a wound culture that only grew one colony of Candida. A retrospective series evaluating the symptomatology and occurrence of prostatic abscesses suggests that prostatic abscesses should be considered in the differential diagnosis of a young man who presents with fever of unknown origin (22).
Given this patient’s multi-organ involvement, the possibility of a MPA originating from a septic focus of the lungs, which was infected with Burkholderia species, is not out of the question. While this question is unanswered by our microbiological investigation, it is a definite possibility. Melioidosis, a condition of immunosuppression, is an infection with the gram-negative bacterium Burkholderia pseudomallel, and is characterized by the formation of prostatic abscesses in several cases (23). Of cases involving the genitourinary system, the most common manifestation is a prostatic abscess. Interestingly, most of those cases were diagnosed after disease was established in other organ systems, primarily the lung.
Although rare, the immunocompromised patient combined with the extensive use of multiple antibiotics should be a cause for increased awareness and early therapeutic interventions are important to avoid increased morbidity and mortality. Given the patient’s state of immunosuppression and use of antimicrobial agents, perhaps the presentation of a prostatic abscess wasn’t so unusual. What is unusual is the unknown cause. This infection was silent and very difficult to diagnose. This particular group of patients with not only CGD but with other immunosuppressant conditions often require additional imaging studies to reveal the presence of enigmatic sites of infection. The genitourinary system can be a source or target of disseminated infection and early recognition is key. The patient is oftentimes asymptomatic, and often lacks the typical clinical signs and symptoms, but nonetheless the importance of a high index of suspicion cannot be undermined.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walther MM, Malech H, Berman A, et al. The urological manifestations of chronic granulomatous disease. J Urol. 1992;147:1314–1328. doi: 10.1016/s0022-5347(17)37552-3. [DOI] [PubMed] [Google Scholar]
- 2.Soler-Palacin P, Margareto C, Llobet P, et al. Chronic granulomatous disease in pediatric patients: 25 years of experience. Allergol Immunopathol (Madr) 2007;35:83–89. doi: 10.1157/13106774. [DOI] [PubMed] [Google Scholar]
- 3.Ross D, de BM, Kuribayashi F, et al. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–1681. [PubMed] [Google Scholar]
- 4.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 5.Liese J, Kloos S, Jendrossek V, et al. Long-term follow-up and outcome of 39 patients with chronic granulomatous disease. J Pediatr. 2000;137:687–693. doi: 10.1067/mpd.2000.109112. [DOI] [PubMed] [Google Scholar]
- 6.Patiroglu T, Unal E, Yikilmaz A, et al. Atypical presentation of chronic granulomatous disease in an adolescent boy with frontal lobe located Aspergillus abscess mimicking intracranial tumor. Childs Nerv Syst. 2010;26:149–154. doi: 10.1007/s00381-009-1003-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang SM, Shieh CC, Liu CC. Successful treatment of Paecilomyces variotii splenic abscesses: a rare complication in a previously unrecognized chronic granulomatous disease child. Diagn Microbiol Infect Dis. 2005;53:149–152. doi: 10.1016/j.diagmicrobio.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira P, Andrade JA, Porto HC, et al. Diagnosis and treatment of prostatic abscess. Int Braz J urol. 2003;29:30–34. doi: 10.1590/s1677-55382003000100006. [DOI] [PubMed] [Google Scholar]
- 9.Kaltenis P, Mudeniene V, Maknavicius S, et al. Renal amyloidosis in a child with chronic granulomatous disease and invasive aspergillosis. Pediatri Nephrol. 2008;23:831–834. doi: 10.1007/s00467-007-0702-0. [DOI] [PubMed] [Google Scholar]
- 10.Johansen KS, Borregaard N, Koch C, et al. Chronic granulomatous disease presenting as xanthogranulomatous pyelonephritis in late childhood. J Pediatr. 1982;100:98–100. doi: 10.1016/s0022-3476(82)80246-1. [DOI] [PubMed] [Google Scholar]
- 11.Kis E, Verebely T, Meszner Z. Inflammatory pseudotumor of the bladder in chronic granulomatous disease. Pediatr Nephrol. 2002;17:220–221. doi: 10.1007/s00467-001-0775-0. [DOI] [PubMed] [Google Scholar]
- 12.Barese CN, Podesta M, Litvak E, et al. Recurrent eosinophilic cystitis in a child with chronic granulomatous disease. J Pediatr Hematol Oncol. 2004;26:209–212. doi: 10.1097/00043426-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Bauer SB, Kogan SJ. Vesical manifestations of chronic granulomatous disease in children. Its relation to eosinophilic cystitis. Urology. 1991;37:463–466. doi: 10.1016/0090-4295(91)80112-k. [DOI] [PubMed] [Google Scholar]
- 14.Redman JF, Parham DE. Extensive inflammatory eosinophilic bladder tumors in children: experience with three cases. South Med J. 2002;95:1050–1052. [PubMed] [Google Scholar]
- 15.Frifelt JJ, Schonheyder H, Valerius NH, et al. Chronic granulomatous disease associated with chronic glomerulonephritis. Acta Paediatr Scand. 1985;74:152–157. doi: 10.1111/j.1651-2227.1985.tb10940.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Rhenen DJ, Koolen MI, Feltkamp-Vroom TM, et al. Immune complex glomerulonephritis in chronic granulomatous disease. Case report of an eighteen-year-old girl. Acta Med Scand. 1979;206:233–237. [PubMed] [Google Scholar]
- 17.Yamazaki H, Nishi S, Chou T, et al. Two brothers with p47-phox-deficient chronic granulomatous disease associated with end-stage renal failure. Nephrol Dial Transplant. 1995;10:2334–2336. doi: 10.1093/ndt/10.12.2334. [DOI] [PubMed] [Google Scholar]
- 18.Kimpen J, Van Damme-Lombaerts R, Van den Berghe G, et al. Autosomal recessive chronic granulomatous disease associated with 18q-syndrome and end-stage renal failure due to Henoch-Schonlein nephritis. Eur J Pediatr. 1991;150:325–326. doi: 10.1007/BF01955932. [DOI] [PubMed] [Google Scholar]
- 19.Friend JC, Hilligoss DM, Marquesen M, et al. Skin ulcers and disseminated abscesses are characteristic of Serratia marcescens infection in older patients with chronic granulomatous disease. J Allergy Clin Immunol. 2009;124:164–166. doi: 10.1016/j.jaci.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shokeir AA, Dawaba M, Abdel-Gawad M, et al. Prostatic abscess in a child. Scand J Urol Nephrol. 1995;29:525–526. doi: 10.3109/00365599509180040. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig M, Schroeder-Printzen I, Schiefer HG, et al. Diagnosis and therapeutic management of 18 patients with prostatic abscess. Urology. 1999;53:340–345. doi: 10.1016/s0090-4295(98)00503-2. [DOI] [PubMed] [Google Scholar]
- 22.Bhagat SK, Kekre NS, Gopalakrishnan G, et al. Changing profile of prostatic abscess. Int Braz J Urol. 2008;34:164–170. doi: 10.1590/s1677-55382008000200006. [DOI] [PubMed] [Google Scholar]
- 23.White NJ. Meliodosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]