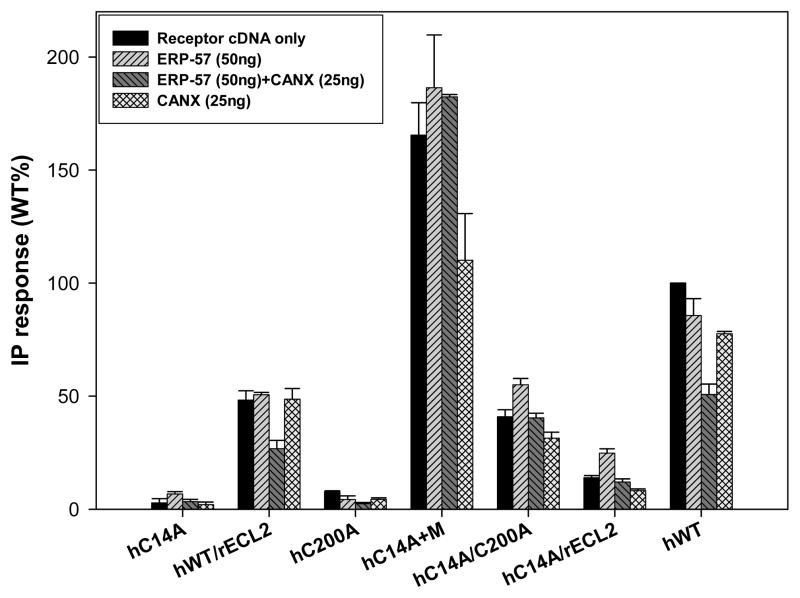
Figure 5.
Cotransfection was performed with the hWT GnRHR as well as hGnRHR mutants in COS-7 cells due to their different IP production. Each one of these proteins was stimulated and their inositol phosphate (IP) production determined without any cotransfected chaperone, with ERP-57 (50ng), CANX (25ng) and ERP-57(50ng)+CANX(25ng). The hWT GnRHR IP production is decreased when cotransfected with ERP-57(50ng) and with CANX (25ng), further restriction is evident when both of these proteins are cotransfected with the hWT GnRHR. Mutations such as hC14A, hC200A and hC14/C200A will prevent disulfide bond bridge formation in the hGnRHR, hence a lower IP production, other mutations such as hC14a+M will increase IP production (“M” is a shorthand notation for receptor with mutations in Leu112Phe/Gln208Glu/Leu300Val/Asp302Glu. (7)). Total inositol phosphate (IP) production of human WT GnRH receptor cDNA (25ng) cotransfected with: empty vector (75ng), ERP-57 (75 ng), ERP-57 + CANX (50ng, 25ng), and CANX alone (25ng). The final amount of DNA was 100ng by adding a complementary amount of empty vector.