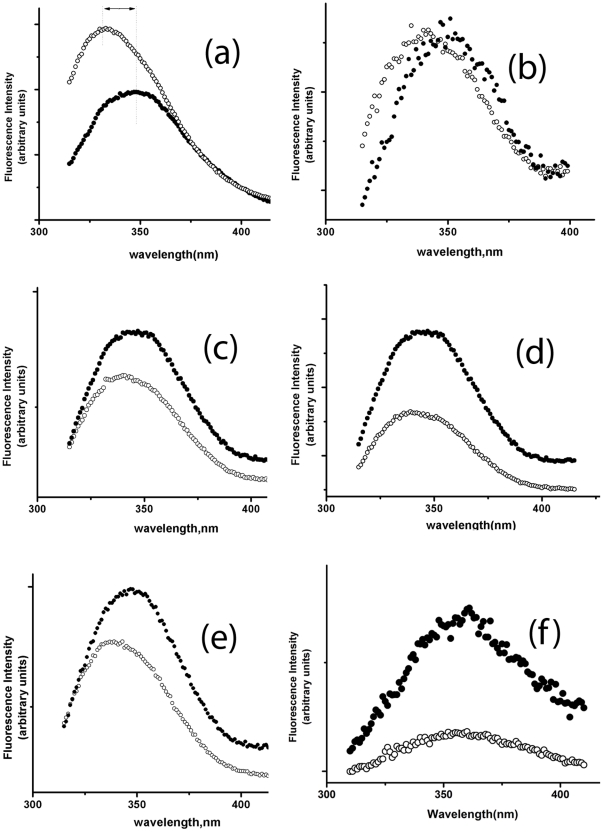
Figure 5. Fluorescence spectra of WT and cysteine mutants of Viperin in folded and unfolded condition.
Fluorescence emission spectra of (a) the WT Viperin, (b) the triple mutant (c) C83A mutant, (d) C87A mutant and (e) C90A mutant (f) NATA in the absence (void circle) and presence (closed circle) of 10 M urea. Fluorescence experiments have been carried out in 20 mm phosphate buffer at pH 7.5. A red shift in the emission spectra is shown by a double headed arrow for the WT protein.