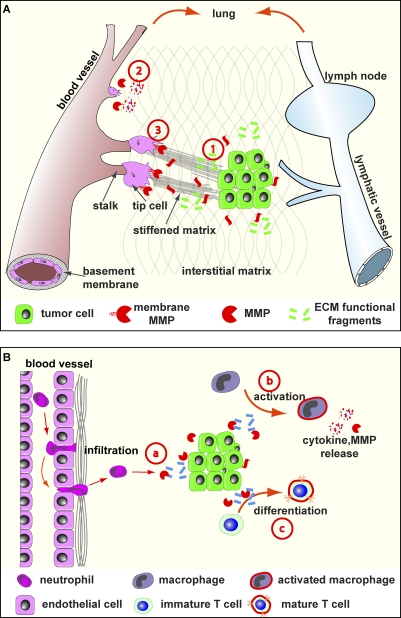
Figure 3.
ECM role in tumor angiogenesis, lymphangiogenesis, and inflammation. (A) Angiogenesis and lymphangiogenesis depend on the ECM. Tumor cells produce various components, including VEGF and angiogenic and antiangiogenic ECM fragments, to regulate blood vessel formation (stage 1). During branch initiation, endothelial cells secrete proteases to break down the basement membrane to grow out (stage 2). The outgrowth process of endothelial branching is propelled by at least two groups of cells: tip cells, which lead the migration toward the angiogenic chemoattractant source, and stalk cells, which depend on the ECM and its derivatives to survive and proliferate to provide building blocks for vessel formation (stage 3). Additionally, ECM components participate in cell migration and other aspects of tubulogenesis of blood vessels. Although details remain unclear, lymphangiogenesis depends on the ECM and, together with angiogenesis, provides routes for cancer cell metastasis and immune cell infiltration. (B) The ECM plays multiple roles in tumor inflammation. In addition to promoting survival and proliferation (not depicted), ECM components function as a chemoattractant to immune cells (stage a). The exact details of how immune cells including neutrophil transmigrate endothelial basement membrane are not clear, though it seems the ECM plays both positive and negative roles in the process. Macrophage activation depends on the ECM to release its potent cytokine signals and protease content (stage b). Further, immune cell differentiation, including maturation of T helper cells, requires participation of ECM components (stage c).