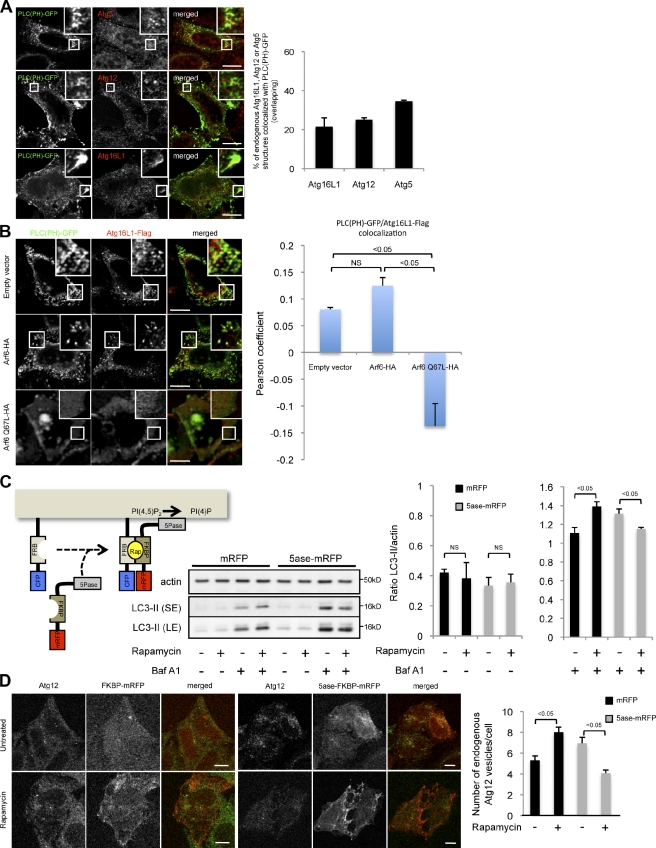
Figure 4.
PIP2 regulates autophagosome formation. (A) Colocalization between PLC(PH)-GFP and endogenous Atg5, Atg12, and Atg16L1. HeLa cells transiently expressing PLC(PH)-GFP for 20 h were cultured in starvation medium for 4 h. Cells were fixed and subjected to immunofluorescence with an anti-Atg5, anti-Atg12, or anti-Atg16L1 antibody. Confocal images of PLC(PH)-GFP and either Atg5, Atg12, or Atg16L1 are shown. The colocalization (overlapping) between PLC(PH)-GFP and Atg16L1-, Atg12-, or Atg5-positive vesicles is shown. (B) HeLa cells transiently expressing PLC(PH)-GFP, Atg16L1-Flag, and either Arf6-HA, Arf6 Q67L–HA, or an empty vector for 20 h were fixed and subjected to immunofluorescence with an anti-Flag antibody. Confocal images of PLC(PH)-GFP and Atg16L1-Flag are shown. The colocalization (Pearson’s coefficient) between PLC(PH)-GFP and Atg16L1-Flag is shown. (A and B) For colocalization, the data are means ± SD. n = 20 cells. (C) HeLa cells transiently expressing CFP-FRB and either mRFP-FKBP or mRFP-FKBP-5ase for 20 h were treated as indicated with 2.5 µM rapamycin for 4 h and 400 nM bafilomycin A1 (Baf A1) for 4 h. Cells were lysed and subjected to Western blotting with the indicated antibodies. The data represent the means ± SD of the LC3-II/actin ratios obtained from three independent experiments. SE, short exposure; LE, longer exposure. (D) HeLa cells transiently expressing CFP-FRB and either mRFP-FKBP or mRFP-FKBP-5ase for 20 h were treated as indicated with 2.5 µM rapamycin for 1 h. Cells were fixed and subjected to confocal microscopy and to automatic counting of endogenous Atg12 vesicles. Representative confocal pictures are shown. The data represent the means ± SD of the number of endogenous Atg12 vesicles/cell obtained from three independent experiments. n = 500 cells in each experiment. Higher magnifications of the colocalizations are shown in the insets. Rap, rapamycin. Bars, 5 µm.