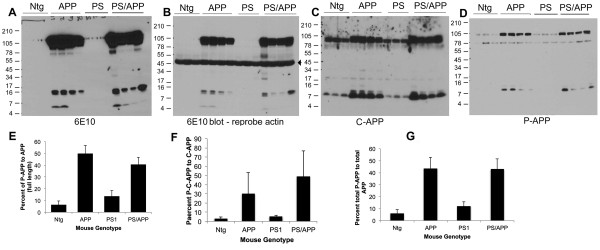
Figure 5.
Increased levels of APP phosphorylation and processing in transgenic mice expressing APP and PS/APP: Equal amounts of proteins from Ntg, APP, PS1, and PS/APP brain extracts were analyzed using 6E10, C-APP, 22C11, and P-Thr668 APP antibodies. A) Shows western blot analysis using monoclonal 6E10 antibody (detect APP, Aβ, and any Aβ containing fragments of APP), B) shows reprobe of the same blot using actin antibody (indicated by arrow) without stripping to show equal amounts of protein loading, C) western blot using a polyclonal C-terminal APP antibody (detects full length and C-terminal fragments of APP), and D) represents the western blot using Thr668 P-APP antibody. Mice expressing APP and PS/APP showed very high levels of full length and C-terminal APP fragments. Aβ levels showed mouse-to-mouse variation probably due to varied expression of the transgenes. Levels of P-APP were significantly higher in both APP and PS/APP transgenic mice and the antibody detected the phosphorylated C-terminal fragment of APP as well. Blots were analyzed using supersignal ECL solution from Pierce. The histograms represent quantitative analysis of P-APP compared to the corresponding counterpart of total APP detected using C-terminal APP antibody: E) percent of full length P-APP, F) percent of P-C-APP (phosphorylated C-terminal fragment), and G) percent of total P-APP compared to total APP.