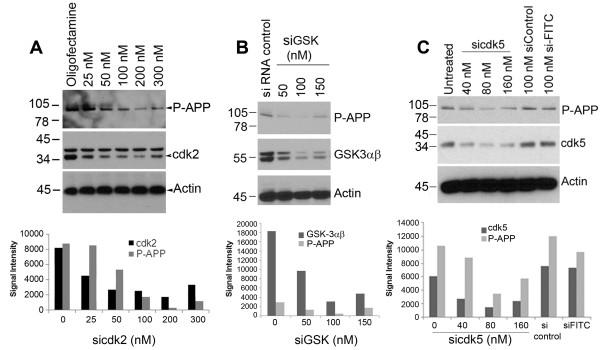
Figure 9.
siRNA to cdk2, cdk5, and GSK-3αβ inhibits serum stimulation-induced APP phosphorylation at Thr668: H4-APP cells plated in serum-free OPTI-MEM were transfected with siRNA to cdk2 (Panel A), GSK-3αβ (panel B) or cdk5 (Panel C) at the indicated concentrations using oligofectamine. After 6 hr serum containing media was added to the cells and samples were collected after 24-48 hr. Cell lysates were western blotted using the corresponding kinase antibodies to confirm downregulation of the respective kinases. Phosphorylation status of APP was analyzed using P-Thr668 APP antibody, and actin was used as a loading control. Down regulation of each kinase was associated with inhibition of serum stimulation-induced phosphorylation on APP. The histograms below each blot show the quantification of the level of the respective kinase and P-APP compared to the levels present in siRNA control transfected cells. The data are representative of one of three independent experiments.