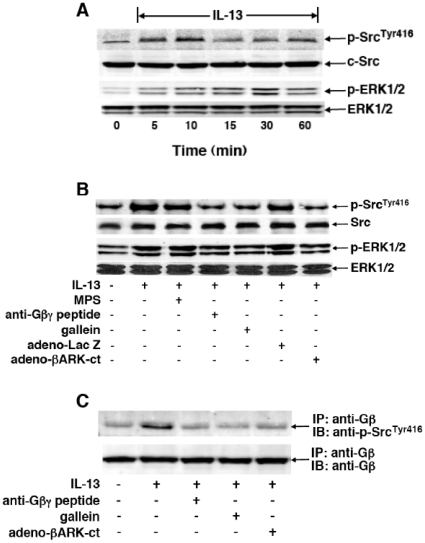
Figure 7. Gβγ signaling regulates IL-13-induced c-Src and ERK1/2 activation in HASM cells.
(A) Immunoblots depicting IL-13-induced transient phosphorylation of c-SrcTyr416 and ERK1/2 in HASM cells, with peak phosphorylation detected at 10 and 30 min, respectively. (B) Immunoblots depicting that, contrasting the lack of effect of pretreatment with MPS alone, IL-13-induced phosphorylation of c-SrcTyr416 and ERK1/2 is suppressed in HASM cells pretreated with either anti-Gβγ peptide (20 µM) or gallein (10 µM). Additionally, in contrast to HASM cells transfected with adeno-LacZ (i.e., negative control), IL-13-induced phosphorylation of c-SrcTyr416 and ERK1/2 is also suppressed in HASM cells wherein Gβγ signaling is inhibited by transfection with adeno-βARK-ct. (C) Western blot depicting interaction of Gβ and c-SrcTyr416 in HASM cells stimulated with IL-13 in the absence and presence of inhibition of Gβγ activation. Following preparation of lysates from untreated and IL-13-treated (50 ng/ml×10 min) HASM cells, Gβ was immunoprecipitated (IP) with anti-Gβ monoclonal antibody, and subsequently immunoblotted (IB) with anti-phospho-c-SrcTyr416 antibody (see Methods). Note, relative to untreated cells, association of Gβ and c-SrcTyr416 proteins was significantly increased in IL-13-treated HASM cells; and formation of this protein complex was suppressed in IL-13-exposed cells that were pretreated with either anti-Gβγ peptide (20 µM) or gallein (10 µM), and also suppressed in IL-13-exposed HASM cells that were transfected with adeno-βARK-ct. The immunoblots shown in A–C are representative from 3–4 experiments.