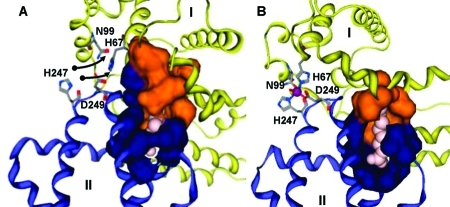
Figure 4.
Different binding modes for (A) medium- and (B) short-chain fatty acids in site FA2 on HSA. Fatty acid molecules are shown in pink. The colored surfaces represent Analytical Connolly surfaces of the residues forming the binding pocket. In both models, the carboxylate headgroup interacts with R257, and the hydrophobic half-pocket in domain II (blue) is formed by residues L250, L251, A254, A258, L283, and L284. (A) HSA with bound MYR, based on PDB entry 1bj5. Three C atoms have been added to the C11 chain resolved in the X-ray structure. Domain I (orange and yellow) contributes to the fatty acid binding site an extended half-pocket comprising residues R10, L14, F19, L22, V23, A26, L66, and Y150. The complete pocket can be formed only if the zinc site (labeled residues) is disrupted. (B) HSA with OCT and Zn2+ (purple) bound simultaneously. OCT is short enough to be accommodated predominantly in the domain II pocket. Hydrophobic residues L14, F19, L22, and L155 form a new half-pocket without disrupting the zinc site.